Topic 14: Further Redox
- Created by: LouiseG
- Created on: 19-04-17 17:33
Reduction and Oxidation
You will know the terms reduction and oxidation from year 1, where you would have referred to them in the context of-
Reduction Oxidation
The loss of oxygen Gain of oxygen
Gain of hydrogen Loss of hydrogen
Gain of electrons Loss of electrons
Oxidation number reduces Oxidation number goes up
You will also know that reducing agents are species which reduce others (i.e. donate electrons), so are themselves oxidised. An example of a good reducing agent is lithium.
Oxidising agents are species which oxidise others (i.e. accept electrons) so are themselves reduced. The best oxidising agent is fluorine.
Metal atoms have a tendency to lose electrons to become cations, i.e. they tend to be good reducing agents. Non-metals, such as the halogens, are often better oxidising agents. They prefer to accept electrons and become anions. (Think ionic bonding from GCSE).
Metals and solutions
When placed in water, metals have some tendency to form ions which go into solution. They tend to do this by losing electrons and becoming positive ions. Some of these positive ions will eventually regain electrons from the surface of the metal, where they build up and return to form part of the metal solid. In this way, an equilibrium is set up, between positive metal ions in solution and the metal solid:
M+ (aq) + e- <----> M(s)
where M could be any metal, and the charge and thus number of electrons could vary.
For each metal, the position of equilibrium will not be in the same place. For example, magnesium's equilibrium lies further left than copper (for the equation as written, with the electrons on the left-hand side). This means magnesium metal has a greater tendency than copper to release electrons (i.e. the backwards reaction is favoured more, compared to copper). Therefore the magnesium set up will have more positive ions in solution, and more electrons on the surface of the metal. The potential difference between the metal and solution is greater.
Standard electrode potentials
But how do you measure this potential difference, between the metal and the ions it has released? If you placed a crocodile clip onto the metal electrode, and another into the solution of ions, you'd end up setting up a new potential difference- that of the metal the clip is made of with the solution. Therefore this absolute difference cannot be measured directly. Instead, a reference electrode is needed, so that different metals can be compared to each other. This reference is the standard hydrogen electrode.The equilibrium that exists in the standard hydrogen electrode is H+ + e- ---> 1/2 H2 (g). The equation does not involve a metal electrode, like with magnesium, so to provide the conduction of charge (so electrons can flow) unreactive platinum is used as the electrode.
Cells with the standard hydrogen electrode
Each of these elements in equilibrium with its ions forms a half cell. This is, unsurprisingly, half a cell, or an electrode. To measure a potential difference, two half cells are connected to form a full cell. They are connected by a salt bridge of potassium nitrate. This allows for the ions to migrate between the half-cells. One of the half-cells will be the negative electrode. This is the cell which has an equilibrium further to the left, i.e. it releases electrons. The other is the positive and accepts these released electrons. Any two half cells could be connected in this way, but to measure a standard electrode potential of a certain half cell, it has to be connected to the hydrogen electrode under standard conditions. There are:
- a pressure of 100kPa, of any gases involved (this is equal to 1 bar).
- A temperature of 298oK
- Each half cell contains a solution of ions, and in the standard set up it needs a 1M concentration of ions, for example, the magnesium half-cell would have a magnesium metal electrode in 1moldm-3 magnesium sulfate (aq). The hydrogen electrode has 1M HCl (aq).
- NOTE that the mass of the metal electrode has no effect on the measured potential difference.
Half cells
This is the example of the set up to measure the potential difference of the magnesium electrode, against the reference hydrogen half-cell. Rather than measuring the potential difference between magnesium and its ions, it is being compared to the potential difference between hydrogen and its ions, by measuring the p.d. between them. As long as all half-cells have been compared to hydrogen in this way, then they can also be compared to each other.
Standard electrode potentials
The high-resistance voltmeter on the external circuit is used to find a value of the electrode potential. This gives an indication of where the position of equilibrium lies for each electrode, relative to hydrogen's. So, by definition, the hydrogen electrode has an electrode potential of 0.00V. Imagine if you connected two hydrogen electrodes together, no current would flow as neither cell would have the better reducing or oxidising agent! Note that this doesn't mean that the standard hydrogen electrode has an equilibrium right in the middle, i.e. the concentration of H+ and 1/2H2 is exactly equal. It has just been set as the convention as 0.0V, as everything else is compared to its equilibrium position. Magnesium's recorded value is -2.37V. This negative sign means its equilibrium lies further LEFT compared to hydrogen, for the equation
Mg 2+ + 2e- <-----> Mg (s)
Therefore magnesium forms the NEGATIVE ELECTRODE as it is the better reducing agent, compared to hydrogen.
To show what is happening when these two half-cells are connected, the ionic half-equations of magnesium and hydrogen are combined. The more negative equation is reversed. (flipped).
Mg (s) ----> Mg2+ + 2e- 2H+ + 2e- ----> H2 (g)
Mg (s) + 2H+ (aq) ----> Mg2+ + H2 (g) Notice the arrows are now showing the reaction going one way, as the equilibrium the half cells had with themselves is disrupted. Magnesium is acting as the reducing agent.
Electrode potential values
Electrode potential values are sign-invariant. This means, that magnesium's half-cell value will always be written as -2.37V, even when the equation is flipped. Additionally, the p.d. value doesn't change if the equation is multiplied through, for example, as on the last slide where the standard hydrogen electrode was multiplied by two to cancel the electrons on both sides.
To calculate the potential difference of any cell created, the equation
more positive standard potential value - more negative standard potential value
is used, where these "electrode potentials" are found by reading off the voltage of the cell when the half-cell required is connected to the hydrogen electrode.
For example, if the magnesium (-2.37V) and copper (+0.34V) half-cells were connected, the reading on the voltmeter would be 0.34 - -2.37 = + 2.71 V
Cell voltages are ALWAYS positive for feasible reactions. Remember to check for double negatives!
Two "negative" cells could be connected, like zinc (-0.76V) and nickel (-0.25V). The negative signs tell you that for both equilibria, the position lies further left compared to hydrogen. However, compared to each other, Zinc's lies further left. Thus it becomes the negative electrode, and nickel the positive. The E-Cell value is -0.25--0.76 = +0.51V
Summary
Remember that electrode potential values are relative to the hydrogen electrode and are found by connecting the half-cell under investigation to the hydrogen electrode and measuring the emf (potential difference).
In summary:
A NEGATIVE value suggests the equilibrium lies more to the left, i.e. the more readily the species on the right loses electrons.
A POSITIVE E-value suggests the species on the LEFT is a better oxidising agent; it acts as the positive electrode and accepts electrons. Thus the equilibrium lies right compared to hydrogen.
It is important to clarify that the "left" and "right" spoken about are to do with this equation:
M+ + e- <---> M or X + e- <----> X- , or basically any half-equation written with the electrons on the left of the equilibrium arrow. This is the convention.
Non-metals and other electrodes
These half-cells don't just need to be metals, and they don't just need to be positive-ion forming species (as seen on the last slide). For example, in a chlorine half-cell, the ionic half equation is
1/2Cl2 (g) + e- ---> Cl- (aq) E cell = +1.36
Chlorine would prefer to accept electrons and form chloride ions compared to magnesium ions, for example. Chlorine is a better oxidising agent than magnesium ions.
But how do you measure these half-cells, if they don't have a metal to form the electrode? The same technique is used as will the hydrogen half cell, in that platinum is used as the metal electrode, and the chlorine gas forms an equilibrium at 298K 100kPa with 1moldm-3 chloride ions in solution.
What about
Fe3+ (aq) + e- ---> Fe2+ (aq) ? Both species are aqueous ions. Here, the solution would contain 1 moldm-3 of EACH ion, for example, 1M Iron(II)sulfate and 1M iron(III)sulfate. The electrode would be platinum covered in porous platinum foil again.
Electrochemical cells
"Electrochemical cell" is the name given to two half-cells connected to each other, with the current flowing between them. You may remember that the half-cells were connected by a salt bridge, which allows the ions of each solution to travel. The salt-bridge is made up of a gel or paper saturated in a salt, often potassium nitrate. This is as potassium ions and nitrate ions are unlikely to interfere with the ions in solution - they don't often form insoluble salts. For example, if potassium chloride was used in the salt bridge, but one of the half-cells contained Ag+ (aq), a precipitate of AgCl (s) would form and the concentration of ions would change. The voltmeter was also described as high-resistance. This is to reduce the flow of electrons around the external circuit, so the maximum potential difference between the two half-cells could be measured, the emf. (if the current is too high through this external circuit, the p.d. measured would be lower than the true value).
Cell diagrams
Cells can be represented in shorthand by cell diagrams, where:
- the oxidised form of each species is written on the inside (The higher oxidation state)
- a straight line is used to indicate a change in phase
- a comma is used to indicate a different species (no state change, like Fe2+(aq), Fe3+(aq) )
- a double-dashed line is used to represent the salt bridge
- The positive electrode is written on the RHS; the only exception being cells containing the standard hydrogen electrode, where hydrogen is always on the left.
- PLATINUM is included if it formed an electrode, like in the hydrogen electrode:

Feasible reactions
If written correctly, the reaction that is feasible (i.e., where the more negative reaction has been reversed) will be the one written from left to right across the cell diagram, as in
the reaction happening in this cell would be
Zn (s) + Cu2+ (aq)----> Zn2+ + Cu(s)
By writing the oxidised species on the "inside" (next to the salt bridge) and putting the positive electrode on the right, you have automatically reversed the zinc equation. Here, zinc is the reducing agent and copper(II) ions are the oxidising agent.
Feasibility
It has already been mentioned that, to write the feasible reaction, you combine two half-equations by writing the more negative one (where the equilibrium lies left, i.e. favours the backwards reaction better) in reverse. The two equations are then multiplied to cancel any electrons and combined. To predict if a given reaction is feasible, you just need to check that it is the more negative reaction that has been reversed. For example:
Cu(s) + 2H+ (aq) ----> Cu2+ (aq) + H2(g) will this reaction happen under standard conditions?
The E-Cell value of the copper electrode is +0.34V, and the hydrogen electrode is 0.0V. Therefore the reaction should have
Cu2+ + 2e- ---> Cu(s)
as the forward reaction, as it is more positive. However, the reaction given has Cu(s) being oxidised to Cu2+. This cannot be a thermodynamically feasible reaction.
In an answer, you could show this by calculating the E-Cell of the "reaction" given.
0.00 - 0.34 = -0.34V. As this is negative, the reaction is not possible.
Feasible or not?
Thus, you'd assume the reaction on the last slide is feasible in the reverse:
Cu2+(aq) + H2(g) ----> Cu(s) + 2H+ (aq)
(The E-Cell value would be +0.34V)
Whilst this is true, the reaction does not occur under standard conditions. This is as the activation energy is too high. Activation energy is to do with kinetics; and is unrelated to enthalpy or entropy or E-Cell values (thermodynamic factors). It is a kinetic factor. Therefore, you'd say the reactants are kinetically stable, as the high activation energy prevents the reaction occurring. Another example is the formation of water, although its E-Cell value; total entropy value and enthalpy value are all favourable, it doesn't happen spontaneously because of the high Ea.
However, non-feasible reactions can also be made to happen. This occurs if the conditions are not standard. For example, if a higher concentration solution was used than 1.0moldm-3, the position of equilibrium could be shifted significantly enough to change which reaction has a position of equilibrium further left or right. An example is the reaction of Manganese(IV) Oxide with HCl.
E Cell, total entropy and lnK
A reaction is feasible (though might not happen) when E-Cell is positive. It is also true that a reaction is only feasible when total entropy, Stotal, is positive. It can be shown that
The emf of a cell is proportional to the total entropy of the reaction.
Furthermore, E-cell is also proportional to lnK, where K is the thermodynamic equilibrium constant (K tells you where the position of equilibrium lies, with a value of K>1 meaning the forward reaction (i.e. formation of the products) is favoured. ) and ln is the natural log.
If E-Cell is positive, then total entropy is positive, then K is greater than 1 (and hence lnk is positive)...
..therefore the reaction is thermodynamically feasible.
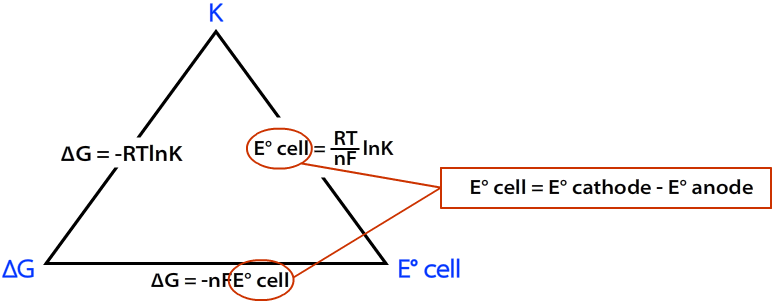 Gibbs energy equations can be used to link these terms
Gibbs energy equations can be used to link these terms
Fuel Cells and Storage Cells
Storage cells
A cell can be recharged by passing a current through in the opposite direction to electron flow generated by the cell. For example, if a cell undergoes the feasible reaction
Cd(s) + 2NiO(OH)(s) + 2H2O(l) ---> Cd(OH)2 (s) + Ni(OH)2 (s)
under standard conditions, to recharge the cell the reaction would be reversed.
Fuel cells
You may remember that a fuel is a substance that can react with oxygen to release energy. In a fuel cell, then, the voltage is generated by the reaction of a fuel with oxygen. One example is the hydrogen fuel cell.
Hydrogen fuel cell
The hydrogen-oxygen fuel cell contains two electrodes coated with platinum, which acts as a catalyst, with an electron flow between them. This is the current generated by the cell. Between the electrodes there is an electrolyte, which transfers ions between the two electrodes, and it can be either acidic or alkaline.
Acidic electrolyte
In an acidic hydrogen fuel cell, it is hydrogen ions (H+) which are the migratory ions across the electrolyte. They pass through a proton exchange membrane, which makes sure no unwanted ions accidentally pass the other way.
Hydrogen fuel is supplied to the negative electrode, where this reaction occurs:
H2(g) ----> 2H+ + 2e-
The hydrogen ions formed cross to the positive electrode, where they react with the oxygen provided:
1/2 O2 (g) + 2H+ (aq) + 2e- ---> H2O (l)
Acidic electrolyte
Combining the two half equations gives
2H2 (g) + O2 (g) ---> 2H2O (l)
Alkaline fuel cell
Alkaline fuel cell
Another alternative electrolyte is the alkaline fuel cell. OH-, rather than H+, ions are involved.
At the negative electrode, H2(g) reacts with OH- ions, forming water.
H2 + 2OH- ---> 2H2O + 2e-
This water diffused to the positive electrode, where oxygen reacts with water:
1/2 O2(g) + H2O + 2e- ---> 2OH-
These OH- ions would then migrate to the negative electrode to react with the H2(g).
Again, adding the two equations gives the same overall equation, H2 (g) + 1/2O2 ----> H2O.
Advantages and disadvantages of the hydrogen fuel
Advantages
- Fossil fuels don't need to be used directly for the fuel
- The only by-product is water, which is not considered a pollutant - no NOx, CO2 or CO is formed
- They are more efficient than fossil-fuel burning engines, and lighter
Disadvantages
- Hydrogen is explosive so difficult to store
- The hydrogen is likely sourced from a non-renewable source (like the cracking of crude oil) therefore the process is not entirely sustainable or carbon neutral
- It is difficult to transport the hydrogen gas; it is not easily compressed (see explosive!) and suitable metals for adsorbing or absorbing the gas are yet to be found.
Redox titrations
Redox titrations are titrations that involve species changing their oxidation number. For example, potassium manganate(VII) (MnO4-) is an oxidising agent and can be used to determine the quantity of a reducing agent in a substance, such as Fe2+ ions. It is also useful because reactions with potassium manganate (VII) are self-indicated. This is as the substance is a very dark purple, but forms a very pale (effectively colourless) pink ion, Mn2+, when reduced. During a titration, the first appearance of a permanent pale pink colour indicates the end point of the titration. This is not due to the Mn2+, but instead due to the first drop of excess MnO4- colouring the mixture.
Potassium manganate (VII) has to be titrated in acidic conditions. This is as, under alkaline conditions, a brown precipitate of Mn2O (manganese(IV) oxide) forms. This would interfere with, and obscure the end point of the titration. The reaction for the reduction of MnO4- is:
MnO4- + 8H+ +5e- ---> Mn2+ + 4H2O
Fe(II) determination
Potassium manganate(VII) is useful for determining the concentration of Fe(II) in a substance, for example how much iron(II)sulfate is in an iron tablet. (This is a core practical). You can probably see that the two react in a 1:5 ratio, as potassium manganate(VII) is reduced by 5e- :
MnO4- + 8H+ + 5e- ---> Mn2+ + 4H2O and Fe2+ ----> Fe3+ + e-
Combining them gives
MnO4- + 8H+ 5Fe2+---> Mn2+ + 4H2O + 5Fe3+
The colour change at the end point is colourless to pale pink, as the Fe3+, Fe2+ and Mn2+ ions are all taken to be colourless at such dilute concetrations.
Calculations for redox titrations are very similar to acid-base. Firstly, the moles of potassium manganate are found by titre/1000 x concentration (volume in dm-3 x conc in moldm-3 = moles). The moles of iron(II) are found using the 1:5 ratio. The moles of iron(II) in the original solution are found, if for example 25cm3 samples had been taken from a 250cm3 volumetric flask then multiply the answer by 10. The mass of iron, and the percentage by mass of iron in the tablet, can be found by moles x 55.8 (55.8 is the Mr of iron).
Ethandioic acid
Another substance's concentration that can be found using potassium manganate(VII) is ethanedioic acid. This is like ethanoic acid but with two carboxylic acid groups.![]() Ethanedioic acid is oxidised to CO2 in this reaction
Ethanedioic acid is oxidised to CO2 in this reaction
H2C2O4 + ---> 2CO2 + 2H+ + 2e-
Therefore it reacts in a 5:2 ratio with potassium manganate(VII).
Ethanedioic acid is toxic and is the poison that makes rhubarb leaves inedible. Therefore, any titrations carried out with it need appropriate safety measures such as using gloves, pipette fillers and wearing goggles. The reaction vessel must also be heated as the reaction is very slow. However, the reaction speeds up as it proceeds as the reaction is autocatalysed (catalysed by itself) - a product of the reaction, the Mn2+ ion, catalyses the reaction.
A very similar titration can be carried out with the ethandioate ion, (COO-)2 :
2MnO4- + 16H+ + 5C2O42- ----> Mn2+ + 10CO2 + 8H2O
Iodine and thiosulfate
Thiosulfate, S2O3 2-, reduces iodine to iodide ions. The reaction is
2S2O3 2- + I2 ---> S4O6 2- + 2I-
Unlike with potassium manganate(VII), this is not self-indicated as the colour change is difficult to see (brown->pale yellow -> colourless). Instead, as the brown colour of the iodine begins to fade, freshly prepared starch solution is added. This is a blue-black colour in Iodine, I2, but colourless in iodide.
The starch isn't added at the beginning because it would absorb some of the iodine and reduce the accuracy of the titration.
This reaction is useful for determining the concentration of different species in other reactions. For example, if the percentage of copper in brass was being determined, then the brass could be dissolved in sulfuric acid and this reaction carried out:
2Cu2+ + 4I- --->2 CuI + I2
Where the 4I- is from an excess of potassium iodide, KI. The resulting solution can then be titrated with thiosulfate, to calculate the amount of iodine formed and therefore the amount of Cu2+ in the original solution.
Related discussions on The Student Room
- Electrochemistry help »
- Inorganic Chemistry (AQA) - A level »
- A-level Chemistry Study Group 2022-2023 »
- Need Help on an Electrochem Q »
- Edexcel A-Level Chem Paper 1 Advanced Inorganic and Physical Chemistry [Exam Chat] »
- Chem a level - electrochemistry »
- Redox chemistry: help me understand the answer »
- Question »
- Electrochemical cell diagram: when do you add H+ to the solution? a level chem »
- Urgent OCR Chemistry Help »
Comments
No comments have yet been made