Topic 15: Transition Metals
Revision cards for new spec (2015) Edexcel A Level Chemistry, topic 15. (Other topics are also available) Good luck in your exams!
- Chemistry
- Transition metalsBonding & shapesElectrode potentialsRedoxReactionsThe Periodic Table
- A2/A-level
- Edexcel
- Created by: LouiseG
- Created on: 18-01-17 16:11
Electronic configurations
The transition metals are located in the central block of the periodic table - the d block (the block where the last electron was added in a d orbital). In this topic we only look at Period 4; the first row of transition metals. These elements all have certain physical and chemical characteristics and properties that they mostly share:
- They are hard, lustrous and sonorous
- Have high melting and boiling points
- Act as catalysts
- Form coloured ions and compounds
- Form ions with varying oxidation numbers
- Form complexes
- Form stable ions with incompletely filled d-subshells.
It is the last point which is actually the definition of a transition metal - "A d-block element that forms stable ions with an incompletely filled d subshell".
For period 4, this will be elements with ions that contain 3d1 through to 3d9.
Zinc and Scandium are not transition metals, although they are d block elements. This is as their only stable ions - Zn2+ and Sc3+ - both have either a full or empty 3d subshell, 3d10 and 3d0 respectively.
Oxidation Numbers
It is this defining property of transition metals that gives them so many of their characteristics, such as their colour; ability to act as catalysts; and variable oxidation states.
Many of them can loose a variable number of electrons from the d subshell when forming ions, so have different oxidation states.
Of Period 4 transition metals, the element that exhibits the highest oxidation number is manganese, which has the MnO4- ion (Manganate(VII) ). In fact, the highest oxidation number of an element increases along the period up to manganese, as the number of electrons available for bonding increases by one each time.
However, after manganese, the highest ON an element usually has drops back down. This is as the effect of an increased nuclear charge now becomes the overriding factor, so the extra electrons are held more "tightly" and thus less likely to be available for bonding.
Higher oxidation states also don't tend to be found in simple ions (like Cu2+ is, for example); but instead tend to be complex ions which involve electronegative elements, especially oxygen (Like the MnO4- example, or CrO4 2- in chromium - where chromate has an ON of +6)
Copper and Chromium - electronic structures
The transition metals' electronic structure can be worked out using spdf notation, where electrons fill in the order
1s2 2s2 2p6 3s2 3p6 4s2 3d10 .....For period 4, this can be written as [Ar] 4s 3d
As uncharged atoms, they all have full 4s orbitals, except copper and chromium which are half-filled, and have the structures:
Chromium - [Ar] 4s1 3d5 Copper - [Ar] 4s1 3d10
So they have half-filled and fully filled 3d-orbitals instead. This is a more stable structure. Furthermore, by having only 1 electron in 4s rather than a pair, repulsion is reduced.
Ligands
When we think of ions in solution, we may imagine them as Mg2+ (aq); or Fe3+ (aq). Whilst this is to a degree correct, for transition metals, it is better to consider the ligands that interact with the ion as well:
A ligand is a species that uses a/some lone pair(s) of electrons to form a dative covalent bond to a metal cation.
For Fe3+ in pure water, for example, it will be bonded to water ligands - this is written [Fe(H2O)6]3+ , where this overall structure of metal-and-ligands is called a complex ion (or just 'complex' if there's no overall charge)
Transition metals form these kinds of bonds as they tend to have smaller radii than their non-transition metal (e.g. alkali metal) alternatives in the same period. This gives them larger positive charge densities, so they'll attract electron-rich species with lone pairs, such as water or hydroxide ions, which will form dative or coordinate bonds.Usually, there will be 6, and the structure of the complex is called octahedral: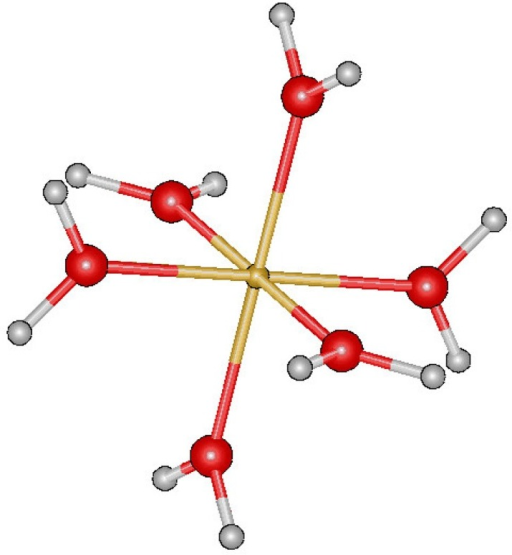
Complexes
Complexes are shown in square brackets, with the overall charge on the outside:
[Cu(H2O)6]2+
There are several parts to the complex and naming it:
- Ligands. These are the species that appear in brackets after the metal ion. The most common are water(aqua) ; hydroxide (hydroxo) ; ammonia (ammine) ; Chloride (chloro-). In brackets is the name given to these ligands in the complex. Note those with a negative charge end in -o. Ligands can be neutral or negative. They just need a lone pair.
- Coordination number. This is the number of coordinate bonds being made to the metal ion. (In monodentate ligands, number of ligands = number of bonds - this is dealt with later). Usually, this number is 6, and the shape is octahedral. It could also be 4 (tetrahedral or square planar) or 2 (linear). This depends on the size, amongst other factors, of the ligand.
- Oxidation number. This is the oxidation state the metal ion is in, which is not the number outside the brackets! (though they can be the same, if all the ligands are neutral).
- Charge. This is the number outside the brackets, and is the overall charge on the complex. It might be 0, for example: if you had Cu2+ and 2OH- ligands (as well as 4 neutral H2Os); the net charge would be neutral.
Naming Complexes
Complexes are named in this order:
- The number of ligands, in di- tetra- hexa- form
- The name of the ligand, in alphabetical order: for example, ammine before aqua before chloro
- The name of the metal ion. If the overall charge is negative, this should be the Latin name, which is often like the metal with the end replaced by -ate: such as cuprate (copper) or chromate(chromium); with ferrate for iron.
- The oxidation number on the metal ion (NOT the charge!)
For example:
[FeCl4]-
Is Tetrachloroferrate(III) -The 3+ oxidation state of Fe and the four Cl- gives this complex a -1 charge overall, (3 -4 = -1 ) so it is named as "ferrate" rather than "iron".
[Cu(NH3)4(H2O)2]2+
Is Tetraamminediaquacopper(II)
* note - Hexaaqua, tetraammine, etc. - with two double a's - is a correct spelling. Also, an ammine is different to an amine, so remember the spelling!
Colour
Transition metals form coloured complexes. Whereas many ionic solids, such as NaCl, are white and crystalline and form colourless solutions, transition metal complexes will exhibit a broad range of colour. Note that Zn and Sc, as non-transition metals, form white/colourless compounds.
The reason is that transition metal ions have incompletely filled d subshells. When approached by a ligand (such as water in an (aq) solution), the orbitals split into two energy levels, with an energy gap between them. There is always a space in an orbital (incompletely filled orbitals); which means an electron in the lower energy level can absorb light energy and be promoted to the space in the higher energy level. The frequency of light absorbed is very specific to the size of the energy gap, where E=hf - i.e., only a certain colour will be absorbed. Therefore the light passing through (transmitted) the complex will have disproportionately high amounts of the complementary colour in the spectra, thus it appears this colour - a compound that absorbs blue light will appear orange, for example.
Complex Shapes
It has already been mentioned that most complexes form octahedral shapes, with 6 coordinate bonds. The bond angles and shapes are recognisable from Year 1, however, to work out shapes electons in the 3d subshell are ignored - just count the number of coordinate bonds. So the angles are-
Octahedral - 90o (6 bonds)
Tetrahedral - 109.5o (4 bonds)
Square Planar - 90o (4 bonds also, rarer case) (You wouldn't have seen this in Year 1)
Linear - 180o (2 bonds)
Complexes can be described as having coordination depending on the numbers of coordinate bonds formed, for example, an octahedral complex will have 6-fold coordination.
Tetrahedral complexes may form because their ligands are too big for 6 to fit. The common example is chloro- : you find [CuCl4]2- and [CoCl4]2- ; which are tetrahedral, but not Cl6. The chlorine ligands are much larger than water. Their negative charge also increases repulsion.
Cis Platin
Platin is a square planar complex, which means it has four ligands - two chloride, two ammonia - arranged as the corners of a square (90o) around a central platinum(II) ion. It has two stereoisomers:
- Cis platin, where similar groups are next to each other, i.e. on the same "side" of the square
- Trans platin, where the similar groups are across the square (i.e. on the diagonals).
Although they look similar, cis-platin is actually useful as a cancer drug - it inhibits cancer cells from dividing by preventing the separation of their DNA - trans-platin has no known medical use and is toxic. Therefore, when supplied as a drug, only the cis-isomer can be dispensed.
One of the reasons that this is square planar and not tetrahedral is because platinum is not a Period 4 TM and acts differently. In other molecules (XeF4) two lone pairs on the central atom repulse each other and force the shape to become square.
Denticity
Denticity refers to how many coordinate bonds a ligand can make to the metal ion. All the examples so far have been monodentate - they only form one bond per ligand (i.e. use just one lone pair, even if they have more (such as H2O) - there might not be space to use both, as lone pairs repel each other and therefore need to be separated to "bend" the molecule.)
Multidentate ligands form more than one dative bond per ligand - for example, bidentate ligands can form 2 coordinate bonds using their lone pairs.
One bidentate ligand is 1,2-diaminoethane; like ethane with two amino groups (NH2) at either end rather than hydrogens. The two nitrogens at either end of the molecule have lone pairs that can be used to form coordinate bonds. For example, 3 1,2-diaminoethane ligands (known as 'en' in notation) could bond to a central metal ion, forming a octahedral shape (The shape refers to the number of bonds, not ligands):Another multidentate ligand is EDTA 4-, which can form 6 bonds, using lone pairs in two nitrogens, and :O- groups.
Stability and entropy
As mentioned previously, smaller molecules tend not to be multidentate ligands, because their lone pairs repel each other and won't point in the same direction to form coordinate bonds. Larger molecules, like the EDTA 4- ion, can fold around themselves, as they have more space. (This is an octahedral structure).
Complexes in general will replace monodentate ligands with multidentate ligands. This is as this reaction would increase the number of molecules, and thus entropy would increase (which is needed for a feasible, spontaneous reaction). For example:
[Cu(H2O)6]2+ + EDTA 4- ---> [Cu(EDTA)]2- + 6H2O
The number of species has increased from 2 to 7, so increases. Complexes with multidentate ligands are more stable.
Haemoglobin
Both oxygen and carbon monoxide can act as ligands.
Haemoglobin is used in oxygen transport. The protein ('globin') part is made up (amongst other things) of four haem groups, which include a Fe(II) ion. This can form a dative bond with oxygen in the lungs, which is released where needed. (reversible reaction)
However, if carbon monoxide is inhaled, it forms a much stronger bond, which is essentially a non-reversible reaction of:
Haemoglobin + carbon monoxide --> carboxyhaemoglobin
This means the body does not get the oxygen it needs and this can kill you by suffocation.
Reactions of Complexes
Complexes can undergo several reactions, including:
- Redox - the oxidation number of the transition metal changes, like Fe2+ ---> Fe3+ .
- Acid-Base - by the Bronstead-Lowry definition, when there is a transfer of H+ ions (protons) between the ligands in the complex and another species
- Ligand exchange - when one ligand is replaced by another (like EDTA replacing H2O)
- Coordination Number change - this often accompanies ligand exchange, for example chloride ions replacing H2O in [Cu(H2O)6]2+ would change the coordination number from 6 to 4
These changes can all also cause a colour and state change, which you will have to learn (!) for Copper, Chromium, Cobalt, Iron (II and III), and Vanadium
The good news is, some of them do follow a set of patterns...
Copper
When we think of copper, we may think blue - such as copper(II) sulfate solution. This is, in fact, the complex ion, [Cu(H2O)6]2+ - hexaaquacopper(II). It is a pale blue solution.
ACID-BASE reactions
If NaOH(aq) is added, the blue solution becomes a uncharged blue precipitate. This reaction is:
[Cu(H2O)6]2+ +2OH- ---> [Cu(OH)2(H2O)4] + 2H2O . This looks like ligand exchange - now we have two hydroxo- ligands - but in fact it is acid base, and the hydroxide ions have taken two H+ ions to become H2O. This can be seen clearly by using another Bronstead-Lowry base, NH3:
[Cu(H2O)6]2+ +2NH3 ---> [Cu(OH)2(H2O)4] + 2NH4+
You can see it is exactly the same reaction, except ammonium forms. You'd see the same changes. However, if excess ammonia is added, a LIGAND EXCHANGE reaction does occur:
Cu(OH)2(H2O)4] + 4NH3 ----> [Cu(NH3)4(H2O)2]2+ + 2H2O + 2OH-
This is a very dark blue solution, Tetraamminediaquacopper(II).
Copper (2)
COORDINATION NUMBER CHANGE / LIGAND EXCHANGE
When HCl is added to copper sulfate solution (which we know exists as [Cu(H2O)6]2+ ) a reaction occurs:
[Cu(H2O)6]2+ + 4Cl- <---> [CuCl4]2- +6H2O
This is both ligand exchange and coordination number change, as the larger chloride ligands can only fit 4 around the copper cation. The solution, tetrachlorocuprate(II), formed is yellow, and will look green half way through (blue + green).
You can also see the reaction is reversible, so will often appear green as it's a mix of both species.
- This reaction has no change in oxidation number, although the overall charge changes. Remember that for naming, this makes copper become cuprate.
In conclusion, in terms of remembering colour, copper in general forms blue solutions and precipitates (except as tetrachlorocuprate(II) ). Remember the sequence blue solution ---> blue precipitate ---> dark blue solution . This solution-precipitate-solution sequence will come up again...
Cobalt
Cobalt(II) undergoes very similar reactions to copper(II) (with a few small annoying differences!).
[Co(H2O)6]2+ is a pink solution. It will also, like copper, react with both ammonia and hydroxide ions to form an uncharged species, again a blue precipitate - [Co(H2O)4(OH)2].
[Co(H2O)6]2+ + 2OH-/2NH3 ---> [Co(H2O)4(OH)2] + 2H2O/2NH4+ (this fades to a pink ppt)
And, again, excess ammonia can cause another reaction that OH- ions can't - HOWEVER - it is slightly different this time - 6 AMMONIA are substituted:
[Co(H2O)4(OH)2] + 6NH3 ---> [Co(NH3)6]2+ + 4H2O + 2OH-
A brown solution forms, which becomes yellow as it's oxidised by the air to [Co(NH3)6]3+] This is a redox reaction.
Cobalt also undergoes a similar chloride substitution reaction, with a pink --> blue (aq) reaction:
[Co(H2O)6]2+ + 4Cl- --> [CoCl4]2- + 6H2O
Iron(II)
Iron (II), like cobalt and copper, undergoes a reaction whereby a Bronstead-Lowry base accepts protons from its complex to form a neutral species:
[Fe(H2O)6]2+ + 2OH- /2NH3 ---> [Fe(H2O)4(OH)2] + 2H2O/2NH4+
A green solution forms a green precipitate. You have probably noticed that in this kind of reaction, the number of OH- or NH3 molecules that react is always equal to the charge on the complex, i.e. it leaves a neutral species by taking two protons. It is also always a PRECIPITATE.
There is NO second reaction with excess OH- or NH3.
[Fe(H2O)4(OH)2] ---> [Fe(H2O)3(OH)3]
The tetraaquadihydroxoiron(II) can be oxidised to triaquatrihydroxoiron(III) by the air, turning the green precipitate to a brown precipitate. This, of course, is a redox reaction.
When thinking Fe(II) think GREEN.
Iron(III)
Iron(III) does exactly what we'd now expect - it can react with a base - NaOH or NH3 - to form a neutral, solid, complex.
[Fe(H2O)6]3+ + 3OH-/3NH3 ---> [Fe(H2O)3(OH)3] + 3H2O/3NH4+
A brown-yellow solution forms a brown precipitate (the same one on the last slide).
Notice that now 3OH- and 3NH3 are reacting, as the TM now has a 3+ charge.
No more reactions occur (it is fully oxidised).
Fe(III) - think brown.
Chromium(III)
Chromium does have some of the same reactions, however as it has higher oxidation states (up to VI) it has additional reactions, that can be written using electrode potentials (Topic 14).
([Cr(H2O)6]3+ is weird because it can appear both green and violet, depending on the presence of other ligands in solution, though I will refer to it as green from now on).
[Cr(H2O)6]3+ + 3OH-/3NH3 ---> [Cr(OH)3(H2O)3] + 3H2O/3NH4+
This reaction is like that of Fe(II) in terms of the colour. The colour change is a green solution forms a green precipitate. It is an acid-base reaction.
HOWEVER, this time a further reaction can happen with BOTH OH- and NH3:
[Cr(OH)3(H2O)3] + OH- ---> [Cr(H2O)2(OH)4]- + H2O (+2OH- -----> [Cr(OH)6]3- )
The green ppt redissolves into a green solution, and there is no further change of appearance with more substitution of OH- ions. These are all acid base, however excess ammonia causes a ligand exchange:
[Cr(OH)3(H2O)3] + 6NH3 ---> [Cr(NH3)6]3+ + 3H2O + 3OH- (a VIOLET solution forms)
The fact chromium undergoes these additional reactions can be used to distinguish it from the similar-looking Fe(II), which stops at the green precipitate stage.
Chromate(VI)
There are two forms of Chromium with a (VI) oxidation state you will see -
CrO4 2- and Cr2O72-
CrO4 2- forms when [Cr(OH)6]3- (the final green solution formed from the acid-base reaction with NaOH, last page) reacts with hydrogen peroxide in alkaline conditions. The colour change is, of course, green to yellow:
2[Cr(OH)6]3- + 3H2O2 ---> 2CrO4 2- + 2OH- + 8H2O
Therefore, it is stable in alkaline conditions. However, if it is transferred to acidic conditions, it becomes more stable as the dichromate(VI) ion. The solution becomes orange.
2CrO4 2- + 2H+ ---> Cr2O72- +H2O
Therefore, this reaction is easily reversible by making the conditions alkaline again.
Dichromate(VI) and chromium(II)
The dichromate ion may be familiar from As, where potassium dichromate was used as the oxidising agent to oxidise alcohols to aldehydes, carboxylic acids or ketones. It is always referred to as acidified potassium dichromate as this is the condition the dichromate complex ion is stable in. You may remember the colour change during redox is orange to green.
As well as alcohols, it can be reduced also by acidified Zinc, which itself is oxidised from Zn to Zn2+, and the same colour change is seen:
Cr2O7 2- + 14H+ + 3Zn ---> 2Cr3+ +7H2O + 3Zn2+
It is the chromate(III) which is green; we've already seen this as the green solution [Cr(H2O)6]3+ (but now we will think in terms of just the ions, not ligands, as they're the important parts).
Looking at the E Cell values, you can see that Zn can further reduce the Cr3+ to Cr2+ :
2Cr3+ +Zn ---> 2Cr2+ +Zn2+
The Cr2+ - [Cr(H2O)6]2+ - is a blue solution.
How do you work these out? Using E values from Redox, with the half equations of Zinc//Zn2+ , the various chromate ions and the hydrogen peroxide reduction. Remember that the equation that is more negative is flipped....
Vanadium
Vanadium is somewhat the easiest transition metal to remember changes for, as it has the same colour for each of the hydrated ions in their different oxidation states:
Vanadium (II) , V2+, is purple
Vanadium(III), V3+, is green
Oxovanadium(IV), VO 2+ , is blue - (this is an O.N of 4+)
Dioxovanadium(V) VO2 +, is yellow - (this is an ON of 5+)
It, like chromium, is reduced all the way from 5+ to 2+ by Zn/H+ . You can use E cell values to show why. (The Zn half cell, Zn2+ + 2e- ---> Zn ; is quite negative ( -0.76V), however not as negative as V2+ + 2e- ---> V -1.18 V , so it cannot reduce all the way down to Vanadium solid).
Test yourself on some of the colours of these complexes at https://getrevising.co.uk/revision-tests/transition-metals-colours-test-part-1-1?game_type=quiz and https://getrevising.co.uk/revision-tests/transition-metal-colours-test-part-2-1?game_type=quiz
Heterogeneous Catalysts
Heterogenous catalysts are in a different phase to the reactants they catalyse. Examples include iron in the Haber process, and Vanadium(V)oxide in the contact process. Transition metals are good catalysts as they provide a solid surface for a reaction to occur on, and they have variable oxidation states so can provide alternative pathways with a lower activation energy for the reaction to occur. (Readily accept or donate electrons). Catalysts have to be finely divided or spread as a thin layer over an inert structure, so they have a large surface area and provide more active sites for the reaction to happen on. This is as three steps need to occur in heterogeneous catalysis:
- Adsorption - Reactants become attached to the surface of the catalyst. (Not absorbed into it).
- Reaction - The reaction occurs after the bonds have been weakened.
- Desorption - The newly formed product detatches from the surface.
You need to know how Vanadium(V) Oxide, as V2O5, catalyses the contact process:
SO2 + 1/2 O2 <--> SO3 :this is the process that needs to occur, to make sulfur trioxide.
- SO2 + V2O5 ---> V2O4 + SO3 (+5 ---> +4 ) These are the ON changes of Vanadium.
- V2O4 + 1/2 O2 ---> V2O5 (+4 ---> +5 )
You can see the catalyst is regenerated, and would "cancel" out if you combined the two eqns.
Catalytic Converters

Catalytic converters are used to convert potentially poisionous fumes from car exhausts into safer (but still often pollutant) substances. For example, CO is toxic (causes asphyxiation) and NO causes acid rain.
They have a honeycomb structure to increase surface area, coated in a catalyst of platinum, palladium and rhodium.
One such reaction they catalyse is:
2CO + 2NO ---> 2CO2 + N2
Homogenous Catalysts
Homogenous cataylsts are in the same phase as the reaction reagents, for example two miscible liquids. They are rare in industry, with the disadvantage of the fact they are difficult to separate from the products.
Fe(II) ions can be used to catalyse the reaction of the persulfate ion, S2O8 2-, with iodide ions. The reaction is:
S2O8 2- + 2I- ---> 2SO4 2- + I2
It is slow as the reactants are both negatively charged and repel each other. However, the Fe(II) is positive so can speed it up by attracting the reactant ions:
- S2O8 2- + 2Fe 2+ ---> 2SO4 2- + 2Fe3+
- 2I- + 2Fe3+ ----> I2 + 2Fe 2+
Once again, the catalyst is reformed and "cancels" in the overall equation. Fe(III) may also be the catalyst, and the steps happen in the other order.
Homogenous Catalysts (2)
Potassium manganate (VII) undergoes a reaction with ethandioate ions where autocatalysis occurs - where a product catalyses the reaction. The product is the Mn2+ ion, and as it's produced the reaction's rate increases. It eventually decreases at the end, as the reactant runs out.
2MnO4 - + 5C2O4 2- +16H+ ---> 2Mn2+ +5CO2 +8H2O
This reaction is also slow at the beginning because the species are both negative (high activation energy), and is sped up by the positively charged Mn2+ ions: (combine to cancel the Mn3+)
2MnO4- + 8Mn2+ +16H+ ---> 8H2O + 10Mn3+
5C2O4 2- + 10Mn3+ ---> 10CO2 + 10Mn2+
- The steep part shows where the catayst is speeding up the rate.


Comments
Report