Chemistry F321 Definitions
0.0 / 5
- Created by: WarHorse
- Created on: 19-03-14 21:31
Relative Isotopic Mass (RIM)
Is the mass of an atom/isotope compared to 1/12th of the mass of an atom of C-12
1 of 26
Relative Atomic Mass (RAM, Ar)
Is the weighted mean/average mass of the atoms of all naturally occuring isoptopes of an element, compared to 1/12th the mass of C-12
2 of 26
Relative Formula Mass (RFM)
Is the weighted mean/average mass of the molecules of a substance compared to 1/12th the mass of an atom of C-12Realtive
3 of 26
Relative Molecular Mass (RMM)
Is the weighted mean/average mass of the molecules of a substance compared to 1/12th the mass of an atom of C-12. (The mass of the molecule = the sum of the realtive atomic masses of the atoms in the molecule)
4 of 26
Emperical Formula
Is the simplest whole number ration of atoms in a compound
5 of 26
Molecular Formula
Is the actual numbers of atomes of each element in a molecule
6 of 26
Atomic Number
Is the number of protons in the nucleus of an atom
7 of 26
Mass (Nucleon) Number
Is the number of protons + neutrons in the nucleus of an atom
8 of 26
Isotopes
Are atoms of the same element with a different mass. The isotopes of an element have the same number of protons (and electrons), but different numbers of neutrons
9 of 26
1st Ionisation Energy
Is the energy required to remove 1 electron from each atom in 1 mole of gaseous atoms to form 1 mole of gaseous 1+ ions
10 of 26
2nd Ionisation Energy
Is the energy required to remove 1 electron from each 1+ ion in the 1 mole of gaseous 1+ ions to form 1 mole of gaseous 2+ ions
11 of 26
Nth Ionisation Energy
Is the energy required to remove 1 electron from each (n-1)+ ion in 1 mole of gaseous (n-1)+ ions to form 1 mole of gaseous n+ ions
12 of 26
Ionic Bond
Is the electrostatic attraction between oppositely charge ions
13 of 26
Covalent Bond
Is a shared pair of electrons
14 of 26
Dative Covalent Bond
Is a shared pair of electrons with only one of the atoms contributing both electrons
15 of 26
Electron Pair Repulsion Theory
States that electron pairs repel each other and the number (and type) of electron pairs around the central atom determines the shape of the covalent molecule.
16 of 26
The Order of Repulsion
Lone pair/lone pair > lone pair/bonded pair > bonded pair/bonded pair
17 of 26
Electronegativity
Is the attraction that each bonded atom has for the pair of electrons in the covalent bond
18 of 26
Polar Bond
Is a covalent bond between two atoms with different electronegativities in which the bonded electron pair is drawn close to the more electronegative atom
19 of 26
Polar Molecule
Is a molecule in which the electron density is not equally distributed resulting in areas of high electron density (delta minus) and areas of low electron density (delta plus)
20 of 26
Metallic Bonding
Is the attraction between postitive ions and delocalised electrons
21 of 26
Base
Is a proton acceptor
22 of 26
Acid
Is a proton donor
23 of 26
Salt
Is formed when the proton (H+) in an acid is replaced by either a metal ion or an ammoninum ion (NH4+)
24 of 26
Oxidation
Involves the loss of elctrons or an increase in the oxidation number
25 of 26
Reduction
Involves the gain of electrons or an decrease in the oxidation number
26 of 26
Other cards in this set
Card 2
Front
Is the weighted mean/average mass of the atoms of all naturally occuring isoptopes of an element, compared to 1/12th the mass of C-12
Back
Relative Atomic Mass (RAM, Ar)
Card 3
Front
Is the weighted mean/average mass of the molecules of a substance compared to 1/12th the mass of an atom of C-12Realtive
Back
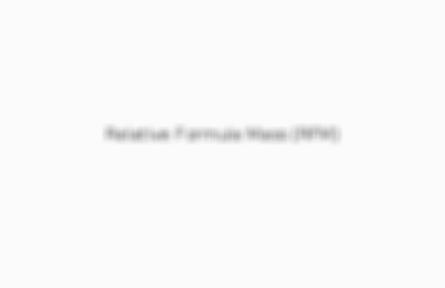
Card 4
Front
Is the weighted mean/average mass of the molecules of a substance compared to 1/12th the mass of an atom of C-12. (The mass of the molecule = the sum of the realtive atomic masses of the atoms in the molecule)
Back

Card 5
Front
Is the simplest whole number ration of atoms in a compound
Back

Related discussions on The Student Room
- Grade Growth Chronicles | From C's to A's (23-24) »
- I enjoy chem practicals more than bio practicals. Is a chem degree right for me? »
- Biochemistry at University »
- Outdoor science jobs? »
- Chemistry vs Natural Sciences »
- What is it like doing chem vs natural sciences at uni? »
- bio, biochem, biomed?? »
- uni modules and how they apply to my course »
- Is my degree unusually difficult or am I bust? »
- Do you need to take A-Level Biology to do well in an Environmental Geography degree? »
Similar Chemistry resources:
4.0 / 5 based on 9 ratings
3.5 / 5 based on 3 ratings
4.5 / 5 based on 27 ratings
0.0 / 5
4.0 / 5 based on 1 rating
4.0 / 5 based on 1 rating
4.5 / 5 based on 15 ratings


Comments
No comments have yet been made