Topic 18B: Amines, amides, amino acids and proteins
For new spec (2015) Edexcel A Level Chemistry, topic 18B (Nitrogen Chemistry). This is called Topic 17.5 in the published textbooks.
- Chemistry
- Amines and azo dyesPolymersReactionsFunctional GroupsAmino acidsAmidesNitrogen chemistry
- A2/A-level
- Edexcel
- Created by: LouiseG
- Created on: 16-02-17 18:10
Amines
 The amines are nitrogen-containing compounds related to ammonia - think of them like ammonia with one or more of its 3 Hs substituted for an aliphatic or aromatic (R) group. The number of Hs substituted tells you whether it is primary, secondary, tertiary etc.
The amines are nitrogen-containing compounds related to ammonia - think of them like ammonia with one or more of its 3 Hs substituted for an aliphatic or aromatic (R) group. The number of Hs substituted tells you whether it is primary, secondary, tertiary etc.
Like ammonia, they (apart from quaternary amine) have a lone pair and three bonding pairs, so have a trigonal pyramidal shape around the N (the quaternary amine would be tetrahedral). This lone pair on the nitrogen in common means they can undergo some similar reactions to ammonia. (For example, they can act as Bronstead-Lowry bases and as ligands).
The amines are named with the suffix -amine; for example, methylamine is the primary amine with 1 methyl group replacing a hydrogen of ammonia. Diphenylamine would have two benzene rings replacing two of the hydrogens of ammonia.
Making Amines from Halogenalkanes
Amines were first met in Organic Chemistry (Topic 6) in Year 1, as the product of the nucleophilic substitution reaction between a haloalkane (RX) and concentrated aqueous ammonia, in a sealed heated tube. For example:
C2H5Cl + NH3 ---> CH2H5NH2 + HCl
The products were a primary amine and hydrogen chloride.
However, the primary amine is still a nucleophile (it has a lone pair) so it can react further:
CH2H5NH2 + C2H5Cl---> (CH2H5)2NH + HCl
To form a secondary amine. This could be repeated again for a tertiary amine, and further again to form a quaternary ammonium salt, such as (CH2H5)4N+Cl-
If ammonia is used in excess, this prevents there being too many ongoing reactions and instead this reaction occurs:
C2H5Cl + 2NH3 ---> CH2H5NH2 + NH4Cl An ammonium halide (white smoke, ionic solid) forms.
Mechanism
This is the mechanism for the reaction on the previous card. Note the subsequent additions would be similar, with an amine nucleophile instead of ammonia. Notice how the first ammonia acts as a nucleophile; and the second as a base (i.e. by accepting the hydrogen ion).
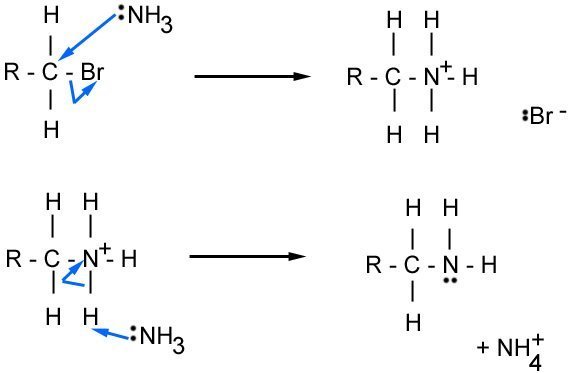
Making Amines from Nitriles
Nitriles (which have the CN functional group) can be reduced to amines. The reducing agent is lithium aluminium hydride, LiAlH4. Like with potassium dichromate in Year 1, you don't need to show the whole agent but instead, represent it as a 4[H] (for the 4 Hs of the agent) Remember reduction is the addition of hydrogen. ([O] was used to represent potassium dichromate as an oxidising agent).
CH3CN + 4[H] ----> CH3CH2NH2
(The red shows where you can think of the Hs as being added).
The conditions are mixed in dry ether. This ensures water is not present to affect the reaction.
Making Aromatic Amines
Phenylamine, the most common aromatic amine, is made from the reduction of nitrobenzene. C6H5NO2 + 6[H] ---> C6H5NH2 + 2H2O
The reducing agents are tin and hydrochloric acid, heated with the nitrobenzene under reflux. The amine formed may be protonated as the conditions are acidic (i.e. NH3+ rather than NH2), therefore a small amount of alkali is added to convert this cation back to its neutral form.
Basic Nature of Amines
Amines act as Bronstead-Lowry bases due to the lone pair on the nitrogen, meaning they can accept protons. Like ammonia, they are soluble in water and produce OH- ions:
CH3NH2 + H2O --> CH3NH3+ + OH-
The amines are less soluble the longer their hydrocarbon chain. They are soluble due to the fact that they can form hydrogen bonds with water (Amines also form hydrogen bonds with themselves, so have quite high BPs. Tertiary amines cannot, they don't have the N-H group to form these bonds with themselves) however as their length increases the hydrocarbon chain interferes with the hydrogen bonding and thus the amines become less soluble.
The longer the aliphatic groups attached to the N in the amine, the more basic it is. For example, propylamine is more basic than ethylamine. This is as the alkyl groups are electron-releasing, so "push" their electrons towards the central N. This makes the nitrogen more negative and its lone pair becomes more available for bonding so it is better at attracting H+ ions - i.e., acting as a base. The nitrogen does not become more electronegative.
However, the benzene ring in phenylamine has the opposite effect. The lone pair on the nitrogen overlaps with the delocalised ring and is accepted into the pi-bond which makes them less available for bonding, and the nitrogen less negative. So phenylamine is a lot less basic than the aliphatic amines.
Reactions With Acids
(The above diagrams show how the lone pair on nitrogen becomes part of the electron ring)
Like other weak bases, amines can react with acids to form ionic salts, the ammonium salts, for example:
CH3NH2 + HCl --> CH3NH3Cl
Methylamine + hydrochloric acid --> Methylammonium chloride
Reactions of Amines - with Acyl Chlorides
Amines can react with acyl chlorides (a homologous series like carboxylic acids, except with a -Cl group instead of -OH: ) to form amides and hydrogen chloride. Amides are another group of organic compounds, which have an amino group (an N bonded with single bonds to hydrogens, aryls or alkyls) attached to a carbonyl (C=O). For example:
(Where the X could be any hydrocarbon chain).
This is an addition-elimination reaction: it is addition as the amine group attaches to the acyl chloride, and elimination as the HCl molecule is eliminated.
Although you don't need to know the mechanism for this reaction, it is helpful to remember the reaction by realising that HCl is always eliminated in acyl chloride reactions: i.e. the chlorine from the acyl chloride and one of the Hs from the amine are eliminated, and you can imagine the remaining species then join together.
Reactions of Amines - with Halogenalkanes
This is the same reaction on card 3: the amines acting as nucleophiles in the reaction with halogenalkanes. These are the possible steps, using butylamine as an example (which is the example given on the specification):
C4H9NH2 + CH3CH2Cl ---> C4H9NHCH2CH3 + HCl
>A primary amine (butylamine) reacts with a haloalkane (chloroethane) to form a secondary amine, buytlethylamine.
C4H9NHCH2CH3 + CH3CH2Cl --> C4H9N(CH2CH3)2 + HCl
>This secondary amine then reacts with more of the chloroethane to form the tertiary amine, which now has two -ethyl groups and one butyl group attached to the N.
C4H9N(CH2CH3)2+ CH3CH2Cl ----> C4H9N+(CH2CH3)3 + Cl-
>The tertiary amine can react one more time and form a quaternary ammonium salt. It has a positive charge overall. Notice this time that HCl isn't formed as there were no more Hs left to be substituted (the ethyl group attached to the lone pair on the nitrogen in a dative bond).
Reactions of Amines - with Copper(II) Ions
It has already been discussed that amines can sometimes behave a lot like ammonia. Like ammonia, they can be ligands, due to the lone pair on the nitrogen meaning they can form covalent bonds with transition metal cations.
These reactions are covered in Topic 15 ( https://getrevising.co.uk/revision-cards/topic-15-transition-metals ) but ammonia will be included for comparison to show how similar the reactions are:
[Cu(H2O)6]2+ + 2NH3 ---> [Cu(H2O)4(OH)2] + 2NH4+ This is the initial acid-base reaction of ammonia with hexaaquacopper(II). The pale blue precipitate of Tetraaquadihydroxocopper(II) forms. If excess ammonia is added, a ligand substituion reaction occurs: [Cu(H2O)4(OH)2] + 4NH3 ---> [Cu(NH3)4(H2O)2]2+ + 2H2O + 2OH-The deep blue solution of tetraamminediaquacopper(II) forms. If we look at the same reactions, but with an amine such as butylamine:
[Cu(H2O)6]2+ + 2C4H9NH2 ---> [Cu(H2O)4(OH)2] + 2C4H9NH3+
[Cu(H2O)4(OH)2] + 4C4H9NH2 ---> [Cu(C4H9NH2)4(H2O)2]2+ + 2H2O + 2OH-
It is the same, except the amine replaces ammonia.
Amides
Amides were introduced as the product of an acyl chloride reacting with an amine. They have an amino group joined to the carbonyl group, where R' and R'' may be hydrogens or other hydrocarbon chains.
Amides are named using their N as a locant to show the groups attached to it. They are known as N-Substituted amides. The part of the name from the amine (R' and R'') are named first, then the carbons attached to the C=O. For example, N-butylethanamide: (make sure to include the C in the C=O bond when counting that carbon chain).
butylethanamide - naming amides, -yl for amine part, -an for carbonyl part. For comparison:
propanenitrile - naming nitriles; the end is -ane (i.e. with an extra e)
ethylamine - -yl end for amines.
Solubility and preparation
Amides are soluble in water as they can form hydrogen bonds with water (they have a N with a lone pair). As with the amines, their solubility decreases as the hydrocarbon chain increases.
The carbon in amides is very d+ (electron deficient) because it is bonded to two electronegative elements, nitrogen and oxygen.
Preparations
Amides can be made from ammonia as well as amines, in the same way: by reaction with an acyl chloride. For example:
CH3CH2COCl + NH3 ---> CH3CH2CONH2 + HCl
Propanoyl chloride + ammonia ---> Propanamide + Hydrogen chloride
You can see that with ammonia, there aren't two parts to the name of the amide formed as ammonia has no extra hydrocarbon chains attached. This is therefore not an N-Sustituted amide.
Polyamides
Polyamides are a kind of polymer made up of repeating amide units, and are formed from condensation polymerisation. This is a type of polymerisation when a small molecule, such as water or HCl, is formed. (You can see that the term condensation comes from the formation of water, but the reaction is called 'condensation' regardless.) Water will form if a carboxylic acid (-COOH) is part of the polymer, and HCl when an acyl chloride is used (-COCl).
Polyamides need two monomers to form, which must have two functional groups per monomer (i.e. a diamine and a dicarboxylic acid/ diacyl chloride). This is as a functional group is needed at either end to allow the reaction to continue:
The carboxylic acid or acyl chloride undergoes an acid/base reaction with the amine, (between the -COOH and the -NH2 part, for example) which eliminates water/HCl. This leaves the CONH of an amide. You can see that at the other end of the chain, the same reaction can keep happening with additional acids/amines and thus a polymer forms.
Amino Acids
The name amino acids gives a clue as to what they are- they contain the amino group (NH2) and the carboxylic acid group (COOH). They are different from amides in that the carbonyl group is separated from the NH2 by another carbon which comes between them:(Amino acids like this are known as 2-amino acids as the N is on carbon 2)
The 'R' group determines which amino acid it is. It does not have to be just a hydrocarbon group, however, it could be a more complex structure. This could include more NH2 or COOH groups.
The fact that the two groups are separated like this means amino acids can behave as both acids and bases (use both functional groups). They are amphoteric.
The amino acids have a chiral centre - they have a carbon with 4 different groups attached (i.e. the NH2, the COOH, the H and the R group). Thus they exhibit optical isomerism. The only exception is glycine due to the fact it has two H groups attached to carbon 2 (i.e. the 'R' is another hydrogen).
Zwitterions and pI
Due to the presence of both an acid and base functional group, amino acids can behave in different ways. They could accept a proton onto the NH2 group to act as a base, or donate from their COOH group. They also exist in a neutral state called a zwitterion, when solid or at a specific pH in solution. This is when both functional groups are ionised - i.e., there has been a transfer of a hydrogen from the -OH in the COOH to the NH2 to give:
The pH at which this exists in solution is called the isoelectric point, or pI. It is specific to each amino acid. A low pI suggests that the amino acid is more acidic and vice versa.
Amino acids exist as their zwitterions in the solid state, forming a lattice. Along with their hydrogen bonds, this gives them high melting points.
Amino Acids at Different pHs
Amino acids are soluble, and at low pHs, an amino acid will exist in its positive form. For example: (two forms of the same reaction are shown)
NH2CHRCOOH + H+ <-> NH3+CHRCOOH or NH2CHRCOOH + H2O <-> NH3+CHRCOOH + OH-
This is the amino acid acting as a base. At a higher pH, it will behave as an acid:
NH2CHRCOOH + OH- <-> NH2CHRCOO- + H2O or NH2CHRCOOH + H2O <-> NH2CHRCOO- + H3O+
(These reactions can also be written with the amino acid in its zwitterion form initially, NH3+CHRCOO-)
The picture is slightly misleading because it suggests that the zwitterion exists at a "middle" pH, i.e. pH 7.00. We know this is not the case; the zwitterion exists at the pI which is different for every amino acid - if the pI is low, the amino acid will exist in its anion form (acting as an acid) for a greater range.
Peptide bonds and polypeptides
Amino acids can react with each other in condensation reactions to form long chains, called polypeptides. Large structures of these are proteins. As amino acids have both basic and acidic parts to them (i.e. they have two functional groups, the NH2 and COOH), they can undergo reactions with themselves to form chains:
The group shown in red is an amide group and the bond between is known as the peptide bond. You can see that at either end these reactions could continue, with different or the same amino acids, forming lengthy chains. The product here is a dipeptide; if another amino acid was added you'd get a tripeptide, etcetera.
This is another example of a condensation polymer. You can probably see that polyamides and peptides can be structural isomers of each other. For peptides, one monomer can have both functional groups (each amino acid has both -COOH and -NH2) and for polyamides two monomers are needed, each with two of the same functional group. You can see then, that the polyamide/peptide could look the same (if the carbon chains are the same), except with the groups swapped around.
Hydrolysing proteins
The reaction on the previous slide can be "reversed" by hydrolysis. In condensation polymerisation, water was eliminated; so it makes sense that to retrieve the original amino acids from their proteins that the reverse is hydrolysis (splitting with water - water is added). This occurs in strong concentrated HCl conditions, for example:
H2NCH(CH3)COHNCH2COOH + H2O + 2H+ ---> H3+NCH(CH3)COOH + H3+NCH2COOH
The dipeptide (made when two amino acids react) of alanine and glycine is split into the two amino acids again (the dipeptide is coloured to show which parts come from what amino acid).
You can see that, as the conditions were acidic, the amino acids formed are in their protonated (positive) forms. If the pH is increased to their pI, they will exist as their zwitterions.
To identify the amino acids that you have hydrolysed, chromatography can be used. This involves spotting the mixture onto chromatography paper, and recording the heights that each one rises to when a suitable solvent is used (this can be used to calculate the Rf value, which can in theory be used to identify the amino acids). The amino acids are first sprayed with a developing agent in order to find their position, as they are colourless.
Related discussions on The Student Room
- A level revision songs »
- HELP!! what does a secondary amide hydrolyze to? »
- Hydrolysis »
- 10 weeks going from C to A* - Alevel »
- OCR Biology Help: »
- Chemistry »
- A level chem question about zwitterions »
- Weak Bases - Names, Properties and Examples »
- Functional Groups Revision »
- Which of the functional groups would result from hydrolysis of a secondary amide? »
Comments
No comments have yet been made