The graph below shows the energy changes that take place during a chemical reaction without an enzyme catalyst.Explain how the presence of an enzyme catalyst would change the shape of this graph.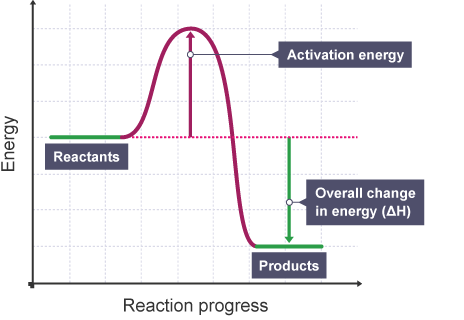
The activation energy (energy needed to start the reaction) would be significatly less.Enzymes reduce the activation energy through a process called catalysis. Enzymes can use the transfer of protons or electrons to the reactants to make them easier to catalyse, therefore meaning they need less activation energy for the reaction to occur.
Suggest if the reaction is endo- or exothermic and give a reason why.
The reaction is exothermic as the energy at the end product is lower than it was initially, which means energy was lost whilst the reaction took place rather than gained like in endothermic reactions.
Comments
No comments have yet been made