Molecules with double bonds
A double bond has 4 electrons as two bonded pairs. To work out the shape of a molecule with double bonds, each double bound is treated as a bonded region, in the same way as a bonded pair. Carbon dioxide has 2 double bonds. They will repel one another to be as far apart as possible, resulting in a linear molecule with a bond angle of 180'.
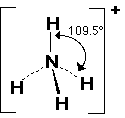
Shapes of ions
The principles discussed can also be applied to any molecular ion. For NH4+, there are 4 electron pairs around the central atom. The shape will be tetrahedral.
Drawing 3d diagrams
- A normal line shows a bond in the plane of the paper
- A bold wedge shows the bond coming out from the plane of the paper towards you
- A dotted wedge shows the bond going out from the plane of the paper away from you
Comments
No comments have yet been made