AQA AS Biology - Biological Molecules
- Created by: charleywalker
- Created on: 20-03-16 18:45
Types of Bonding
Covalent Bonding:
- Atoms share a pair of electrons in their outer shells.
Ionic Bonding:
- Ions with opposite charges attract one another. They are weaker than covalent bonds.
Hydrogen Bonding:
- The elctrons within a molecule are not evenly distributed but tend to spend more time at one position.
- This region is more negatively charged than the rest of the molecule. A molecule with an uneven distribution of charge is said to be polarised (known as a polar molecule).
- The negative region of one polarised molecule and the positively charged region of another attract eachother, forming a weak electrostatic bond between the two. Collectively however, they can form important forces that alter physical properties of molecules, eg water.
Polymerisation and Forming Macromolecules
Key Definitions:
Monomers - Molecules that can join together to form a polymer (a long chain).
Polymer - A long chain made of monomer sub-units.
Polymerisation - The process in which monomers join to form a polymer.
Polymers can be broken down into monomers again, through the process of Hydrolysis.
Hydrolysis takes in a molecule of water during this process and breaks covalent bonds.
Polymerisation is an example of a condensation reaction; so the process of monomers turning into a polymer.
This uses a molecule of water and makes covalent bonds.
Carbohydrates - Monosaccharides
Carbohydrates are made up of a chain of individual molecules; the monomers of carbohydrates are called monosaccharides.
They are sweet tasting and soluble substances.
Examples of Monosaccharides Include:
All monosaccharides are reducing sugars.
The test for a reducing sugar is known as the Benedict's test.
Carbohydrates - Disaccharides
Pairs of monosaccharides can be combined to form a disaccharide.
Glucose joined to Glucose forms Maltose.
Glucose joined to Fructose forms Sucrose.
Glucose joined to Galactose forms Lactose.
When the monosaccharides join, a molecule of water is removed (Condensation Reaction).
The bond that is formed is called a Glycosidic Bond.
When water is added to a disaccharide under suitable conditions, it breaks the glycosidic bond.
This is known as Hydrolysis.
Not all disaccharides are reducing sugars- Sucrose is a non-reducing sugar.
Carbohydrates - Polysaccharides
Polysaccharides are polymers, formed by combining many monosaccharide molecules.
As polysaccharides are very large molecules, they are insoluble.
This makes them very suitable for storage, eg Starch and Glycogen.
However, other polysaccharides, such as Cellulose, are not used for storage but for structural support.
To test for Starch, you add Iodine solution, shake or stir, and observe to see if there is any colour change.
If Starch is present, it will be a blue-black colour.
Starch
Starch has a main role of energy storage:
- It's insoluble, so doesn't affect water potential (so water is not drawn into the cell by osmosis).
- Being large and insoluble, it does not diffuse out of cells.
- It's compact, so a lot can fit in a small space.
- When hydrolysed it forms Alpha Glucose, which is easily transported and readily used in respiration.
- Branched form has many ends, so can be acted on by enzymes simultaneously = glucose monomers are released very rapidly.
Glycogen
Glycogen is found in animals and bacteria.
It has shorter chains than starch but it is more highly branched = more rapidly broken down to form glucose monomers which are used for respiration, which is important as animals have a higher metabolic rate than plants.
Its structure suits it for storage because:
- Its insoluble and therefore does not tend to draw water into the cell by osmosis.
- It doesn't diffuse out of cells.
- It's compact.
Cellulose
Why cellulose is suited to its function of providing support and rigidity:
- Cellulose differs from Starch and Glycogen because it is made up of monomers of Beta-Glucose.
- Cellulose has straight, unbranched chains, which run parallel to one another, which allows for hydrogen bonds to form cross-linkages between adjacent chains.
- Each individual hydrogen bond is weak; but collectively they make a considerable contribution to strengthening cellulose, making it a valuable structural material.
- The cellulose molecules are arranged to form microfibrils, which are arranged in parallel groups called fibres.
- Cellulose is a major component of plant cell walls; providing rigidity to the plant cell.
- It also prevents the cell from bursting as water enters it by osmosis, by exerting an inward pressure that stops any further influx of water.
Lipids
Lipids are a varied group of substances which share the following characteristics:
- They contain Carbon, Hydrogen and Oxygen.
- They are insoluble in water, but they are soluble in organic solvents such as alcohols and acetone.
Roles of lipids include:
- Source of energy; When oxidised, lipids provide more than twice the energy as the same mass of carbohydrate and release valuable water.
- Waterproofing; They are insoluble in water so useful as waterproofing. Both plants and insects have waxy, lipid cuticles that conserve water.
- Insulation; Fats are slow conductos of heat and when stored beneath the body surface help to retain body heat. Act as electrical insulators in myelin sheath around nerve cells.
- Protection; Fat is often stored around delicate organs eg. the kidney.
Triglycerides -Structure
Each fatty acid forms an ester bond with glycerol in a condensation reaction.
Hydrolysis of a triglyceride produces glycerol and three fatty acids.
The glycerol molecule in all triglycerides is the same, the differences in the properties of different fats and oils come from variations in the fatty acids (There are over 70 types).
Triglycerides - Structure Related to Properties
- Triglycerides have a high ratio of energy-storing carbon-hydrogen bonds to carbon atoms and are therefore an excellent source of energy.
- Triglycerides have low mass to energy ratio, good storage molecules because lots of energy can be stored in a small volume.
- They are large, non-polar molecules, so are insoluble in water. This means they do not affect osmosis in cells or the water potential of them.
- As they have a high ratio of hydrogen to oxygen atoms, triglycerides release water when oxidised and therefore provide an important source of water.
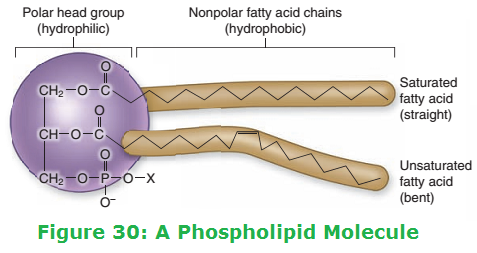
Phospholipids - Structure
Phospholipids are similar to lipids except that one of the fatty acid molecules is replaced by a phosohate molecule.
Fatty acid molecules are hydrophobic, but phosphate molecules are hydrophilic.
Phospholipids - Structure Related to Properties
- Phospholipids are polar molecules (hydrophilic phosphate head and a hydrophobic tail of two fatty acids) so in an aqueous environment, the phospholipid molecules form a bilayer within cell-surface membranes. Results in a hydrophobic barrier forming between inside and outside of cell.
- Hydrophilic phosphate 'heads' help to hold at the surface of the cell-surface membrane.
- Phospholipid structure allows them to form glycolipids by combining with carbohydrates within the cell-surface membrane (important in cell recognition).
Structure of an Amino Acid
Amino acids are the basic monomer units which combine to make a polymer called a polypeptide.
Polypeptides can be combined to form proteins- the same 20 amino acids occur in all living organisms.
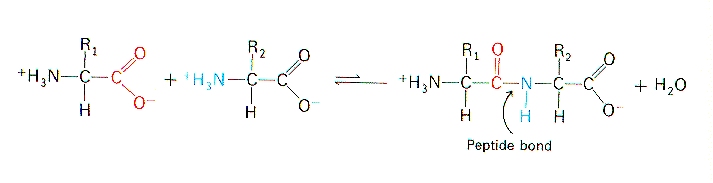
Formation of a Peptide Bond
A condensation reaction joins the 2 amino acids together to form a polypeptide, removing a molecule of water.
The water is made by combining an -OH from the carboxyl group of one amino acid with the -H from the amino group of another amino acid.
The 2 amino acids are then linked by one peptide bond between the carbon atom of one amino acid and the nitrogen atom of the other.
Structure of Proteins
There are 3 bonds holding the tertiary structure: Disulfide cross bridges, ionic + hydrogen.

Enzyme Action
- Enzymes catalyse metabolic reactions (At both cellular level and for the organism as a whole).
- Enzymes have an active site, which has a specific shape, which is the area where substrate molecules bind.
- Enzymes are highly specific due to their tertiary structure.
-Enzymes lower the activation energy of a reaction (energy required to start a reaction) which means reactions can often occur at lower temperatures which speeds up the rate of reaction.
- If 2 substrate molecules need to be joined, being attached to the enzyme holds them close together, reducing any repulsion between the molecules so they can bond more easily.
- If the enzyme is catalysing a breakdown reaction, fitting into the active site puts a strain on bonds in the substrate, so the substrate molecule breaks up more easily.
Related discussions on The Student Room
- Any good youtube channels for Bio + Chem a levels? »
- Do I need to know how to draw structures for carbohydrates? (AQA A Level Bio) »
- exams 2022 »
- A-level Biology Study Group 2023-2024 »
- Paper 3 AQA a Level biology »
- AQA A Level Biology »
- Chemical structures Biology A level »
- For A level Biology students, How reliable/useful are the PMT flashcards? »
- Whats beter revising a whole topic for 1 subject or 2 lessons for 2 subjects A level »
- Access to Science course »
Comments
No comments have yet been made