Chemistry
- Created by: Sophie
- Created on: 23-03-14 11:59
Acid and Alkali Basics
- An acid has a pH lower than 7 and turns blue litmus paper red.
- The lower the pH the more acidic it is.
- An alkali has a pH higher than 7 and turns red litmus paper blue.
- The higher the pH the more alkaline it is.
- A pH of 7 is neutral and does not affect litmus paper but turns universal indicator green.
- Dilute acid and alkalis are irritant.
- Concentrated acid and alkalis are corrosive.
- A base is a substance that reacts with acid and neutralises it.
- Usually: metal hydroxides, metal oxides, metal carbonates, or metal hydrogen carbonates.
- If a base dissolves in water it is an alkali.
- An alkaline base is soluble, all other bases are insoluble.
- Acids are liquids, alkalis feel soapy to the touch
- Acids contain hydrogen ions and alkalis contain hydroxide ions
Neutralisation Reactions
This reaction is called neutralisation. The alkali has neutralised the acid by removing its H+ ions, and turning them into water.
Metal oxide + acid → salt and water
E.g. Copper oxide + hydrochloric acid → copper chloride + water
Metal hydroxide + acid → salt and water
E.g. Sodium hydroxide + nitric acid → sodium nitrate + water
For the two above, the mixture normally heats up a little in the reaction as well.
Metal carbonate + acid → salt and water + carbon dioxide
E.g. Lithium carbonate + nitric acid → lithium nitrate + water + carbon dioxide
Metal hydrogen carbonate + acid → salt + water + carbon dioxide
E.g. Sodium hydrogen carbonate + sulfuric acid → sodium sulfate + water + carbon dioxide
For the two above, The reaction fizzes as bubbles of CO2 are given off.
Uses of Neutralisation Reactions
Soil Treatment - Farming
Majority of plants grow best at pH 7. If soil is acidic or alkaline plant may grow badly. If soil is too acidic it is treated with a base in order to neutralise it. Common treatments use quicklime (calcium oxide) or chalk (calcium carbonate).
Indigestion
We all have hydrochloric acid in our stomach - it helps breakdown food! Too much acid leads to indigestion. So to cure this ailment we need to neutralise the acid with a base such as, sodium hydrogen carbonate (baking soda), or an indigestion tablet.
Insect Stings
A bee sting contains acid. In order to relieve the painful symptoms of the sting we need to neutralise the acid. By rubbing on calamine lotion (zinc carbonate) or baking soda the acid can be neutralised. Wasp stings are alkaline, hence acid is needed to neutralise and remove the painful sting. Vinegar (ethanoic acid) is used.
Waste from Factories
Waste from factories often acidic. If acidic solution not treated and enters rivers can kill fish. Slaked lime (calcium hydroxide) often used to neutralise acid.
Reactions of acids with metals
Metal + acid → salt + hydrogen
E.g. Potassium + phosphoric acid → potassium phosphate + hydrogen
All acids contain hydrogen atoms.
Hydrochloric acid → HCl
Nitric acid → HNO3
Phosphoric acid → H3PO4
Sulfuric acid → H2SO4
Carbonic acid → H2CO3
Atoms and Molecules
- An element is one type of atom, like carbon, gold or chlorine.
- The atoms of some elements do not join up with other atoms. They stay as single atoms.
- When atoms of the same element join together we get a molecule of that element.
- A compound is made when atoms of different elements join together by chemical bonds.
- The properties of compounds are usually very different from the properties of the elements they contain. For example hydrogen and oxygen are both gases at room temperature, but water is a liquid.
- A mixture is made from different substances that are not chemically joined.
- For example powdered iron and powdered sulfur mixed together makes a mixture of iron and sulfur. They can be seperated from each other without a chemical reaction.
- Differences between mixtures and compounds;
- In a mixture, each substance keeps it's own properties, but in a compound it has properties different from the elements it contains.
- In a mixture substances can easily be seperated, in a compound they can only be seperated using chemical reactions.
Reactivity Series
Potassium, Perhaps some lemurs cannot
Sodium, make a zebra in the line
Lithium, concentrate so get pitiful
Calcium,
Magnesium,
Aluminium,
Zinc,
Iron,
Tin,
Lead,
Copper,
Silver,
Gold,
Platinum
Metal and non-metal oxides
Metals react with oxygen in the air to produce metal oxides.
Magnesium + oxygen → magnesium oxide,
Magnesium burns with a bright white flame and produces a white powder, the MgO.
It is slightly alkaline when dissolved in water.
Non-metals react with oxygen in the air to produce non-metal oxides.
Carbon + oxygen → carbon dioxide
It has an orangey-yellow flame. It is slightly acidic when dissolved in water.
Sulfur + oxygen → sulfur dioxide
Sulfur burns with a pale blue flame. It is acidic when dissolved in water.
Metal oxides; solids at room temperature, bases – if they dissolve they form alkaline solutions
Non-metal oxides; usually gases at room temperature, dissolve in water to form acidic solutions Some of these non-metal oxides such as sulphur dioxide and nitrogen oxide are responsible for acid rain. They dissolve in the water in the clouds to form acidic solutions. Acid rain damages rocks and buildings, and harms wildlife.
Seperating Mixtures-Chromotography
Chromatography can be used to separate mixtures of coloured compounds. Mixtures that are suitable for separation by chromatography include inks, dyes and colouring agents in food.
1. Draw a line at the bottom of some filter paper. (Use pencil as it is insoluble and won't dissolve in the solvent).
2. Add spots of different dyes to the line at regular intervals.
3. Put the paper in solvent. (I.e. a beaker of water or ethanol).
4. Make sure dyes aren't touching the solvent as you don't want them to dissolve into it.
5. Place a lid on top of the container so the solvent doesn't evaporate.
6. As the solvent soaks up the paper, it carries the mixtures with it.
7. Different dyes will move at a different rate and form a spot at different places.
8. The series of spots on the paper is called a chromotogram.
Different chromotograms and the separated components of the mixture can be indentified by calculating the Rf value using the equation
Rf = distance moved by the compound ÷ distance moved by the solvent
Separating Mixtures-Filtration and Evaporation
Filtration
Good for separating an insoluble solid from a liquid, for instance sand and water.
You turn filter paper into a cone shape and put it a funnel, then you pour your mixture into it, and the grains of sand will collect in the filter paper as it cannot go through the tiny holes in the paper, and the water will go through.
Evaporation
Good for separating a soluble solid from a liquid for instance copper sulfate and copper sulfate solution.
The solution is put into an evaporating basin. Then it is heated, and the majority of the solvent evaporates away, leaving a concentrated solution and crystals start to form. As the solution is allowed to cool, the solid will come out of the solution and bigger crystals will be there. The crystals can then be collected and allowed to dry.
Separating Mixtures-Distillation
Simple Distillation
To separate a liquid from a solution, for instance water and salty water.
1. The solution is heated. The part of the solution that has the lowest boiling point evaporates.
2. The vapour is then cooled, condesnses and is collected.
3. The rest of the solution is left behind in the flask.
This only works if you have a mixture of liquids with very different boiling points.
Fractional Distillation
Differs from simple only in that it separates a mixture into a number of different parts, called fractions. A tall column is fitted above the mixture, with several condensers coming off at different heights. The column is hot at the bottom and cool at the top. Substances with high boiling points condense at the bottom and substances with low boiling points condense at the top. Like distillation, fractional distillation works because the different substances in the mixture have different boiling points.
The Contact Process-Making Sulfuric Acid
Uses include: Fertiliser production, making detergents, paints, the acid in a car battery
The raw materials are: Sulfur and air
1: Burn sulfur in air to form sulfur dioxide: S + O2 → SO2
Or roast sulfide ores: 2PbS + 3O2 → 2PbO + 2SO2
2. Making sulfur trioxide: 2SO2 + O2 → 2SO3
Temperature: 450 degrees celcius
Pressure: 1-2 atmospheres
Catalyst: Vanadium Oxide (V2O5)
Reaction is reversible and the forward reaction is exothermic (gives out heat).
Increasing the temperature will increase the rate of the reaction, but decrease the amount of SO3 in the equilibrium mixture. 450 is a compromise between the amount of SO3 in the equalibrium mixture and the rate at which sulfur trioxide is formed. 450 gives the best yield of SO3.
3. Making the (concentrated) sulfuric acid: SO3 + H2O → H2SO4
Dehydration
- Removing water from a substance
- Example 1 CuSO4(5H2O) → CuSO4
- Concentrated sulfuric acid
- Blue copper (||) sulfate crystals contain water, five water molecules surround each copper sulfate particle
- Sulfuric acid takes away the water molecules and the copper sulfate becomes white (called anhydrous)
This can be used as a test for water, if you add a couple of drops of solution to the anhydrous copper sulfate, if the crystals turn blue, that means that there is water present in the solution as the copper sulfate is now hydrated
- Example 2 C6H12O6 → C
- Glucose does not contain sugar but does contain H and O which are elements of water
- Sulfuric acid will take away the elements of water leaving you with only carbon
The water removed in these examples dissolved into the sulfuric acid making it more dilute
Making Salts
Salts can be soluble or insoluble
Sodium, potassium and ammonium salts
Nitrates
Most chlorides-bar silver and lead (||) chloride
Most sulfates-bar barium, lead (||) and calcium sulfate
Most carbonates-bar sodium, potassium and ammonium carbonates
Most hydroxides-bar sodium, potassium and ammonium hydroxides
Making soluble salts using acids and insoluble bases
- (1) The required volume of acid is measured out into the beaker with a measuring cylinder. The insoluble metal(only from Mg to Fe in reactivity series), oxide, hydroxide or carbonate is weighed out and the solid added in small portions to the acid in the beaker with stirring.
- (2) The mixture may be heated to speed up the reaction. (Everything apart from carbonates and Mg as they react with dilute acids in the cold. When no more of the solid dissolves it means ALL the acid is neutralised and there should be a little excess solid.
- (3) The hot solution (with care!) is filtered to remove the excess solid metal/oxide/carbonate, into an evaporating dish.
- (4) The hot solution is left to cool and crystallise. Then collect and dry the crystals with filter paper.
Examples of making soluble salts
Copper(II) oxide + sulphuric acid ==> copper(II) sulphate + water
CuO(s) + H2SO4(aq) ==> CuSO4(aq) + H2O(l)
Magnesium hydroxide + sulfuric acid ==> magnesium sulfate + water
Mg(OH)2(s) + H2SO4(aq) ==> MgSO4(aq) + 2H2O(l)
Zinc carbonate + nitric acid ==> zinc nitrate + water + carbon dioxide
ZnCO3(s) + 2HNO3(aq) ==> Zn(NO3)2(aq) + H2O(l) + CO2 (g)
Making insoluble salts-Precipation reactions
A precipitate is a solid
-
An insoluble salt can be made by mixing two solutions of soluble salts in a process is called precipitation.
- The method is quite simple - illustrated above, assuming in this case the insoluble salt is colourless-white.
- One solution contains the 1st required ion, and the other solution contains the 2nd required ion.
- The two solutions of SOLUBLE compounds are mixed together so the INSOLUBLE salt precipitate is formed.
- The precipitated salt can then be filtered off with a filter funnel and paper.
- The collected solid is washed with distilled water to remove any remaining soluble salt impurities and carefully removed from the filter paper to be dried e.g. left out in dry room or warmed in a pre-heated oven.
Worked Example
Silver nitrate + sodium chloride ==> silver chloride + sodium nitrate
AgNO3(aq) + NaCl(aq) ==> AgCl(s) + NaNO3(aq)
Silver nitrate solution contains silver ions and nitrate ions in solution. The positive and negative ions are attracted to each other, but not strongly enough to make them stick together. Similarly the same applies to the sodium ions and chloride ions in the sodium chloride solution.
When you mix the two solutions, the various ions meet each other. When silver ions meet chloride ions, the attractions are so strong that the ions clump together and form a solid. The sodium and nitrate ions remain in solution because the attractions aren't strong enough-these are called spectator ions.
Ionic equation: Ag+(aq) + Cl-(aq) ==> AgCl(s)
The precipitate is impure because of the spectator ions, so the precipitate has to be filtered, washed and dried.
Titration
Titrations allow you to find out exactly how much acid is needed to neutralise a quantity of alkali (or vice versa) In this example I will use sodium hydroxide and hydrochloric acid.
1.The burette is filled with hydrochloric acid
2. A known quantity of alkali (say 25cm3 sodium hydroxide) is released from a pipette into the conical flask.
3. The tap on the burette is turned open to allow the acid to be added drop by drop into the alkali.
4. The alkali contains indicator (phenolphthalein) which is pink in alkali and colourless in acid.
5. When enough acid has been added to neutralise the alkali, the indicator changes from pink to colourless. This is the end point of the titration.
The titration can be repeated using the same amounts of acid and alkali but without indicator.
Pure salt crystals which are free from indicator can then be crystallised from the neutral solution.
Sodium hydroxide + hydrochloric acid ==> sodium chloride + water
NaOH(aq) + HCl(aq) ==> NaCl(aq) + H2O(l)
Redox
Redox
When Iron displaces Copper ions from solution there is an exchange of electrons.
The Iron atoms loses electrons to become positive ions - they have been Oxidised.
Fe(s) → Fe3+(aq) + 3 electrons-
The Copper ions gain these electrons and are Reduced back to Copper atoms.
Cu2+(aq) + 2 electrons- → Cu(s)
The Reduction and Oxidation happen at the same time so we call this a RedOx reaction.
We could say that the Iron has reduced the Copper - making the Iron a Reducing agent.
Or that Copper has Oxidised Iron - making Copper the Oxidising agent.
Generally, whatever is oxidised is the reducing agent (and vice versa). The most reactive metal always ends up being oxidised to an ion. So the most reactive metals make the best Reducing agents. The most unreactive metals make the best Oxidising agents.
Percentage Yield
For a given mass of reactants it is possible to calculate the max possible mass of a certain product that could be made. This mass is called the theoretical yield. The actual yield is the mass of product made when experiment is carried out for real.
Percentage yield = actual yield/theoretical yield x100
Losing product
100% yield means no product has been lost, while 0% yield means no product has been made.
1. You might lose some of the products when you try to seperate them from the mixture after the reaction has finished e.g. filtering and evaporating.
2. The reaction may be reversible.
3. Some of the reactants may form different products from what you want. Some form products which are not useful, these are waste products and may be toxic or difficult to dispose of
Industrial processes need as high a percentage yield as possible, because this:
- Reduces the waste of reactants
- Reduces the cost of the process
State Symbols
SymbolMeaning (s) solid (l) liquid (g) gas (aq) aqueous (dissolved in water)
So for the reaction between sodium and water, this is the symbol equation with state symbols:
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
Reactions of acids with Ammonia
-
Ammonia gas is very soluble in water to form an alkaline solution that can be neutralised by acids to form ammonium salts. All solutions involved here are colourless and all the salts form colourless crystal if the solution is carefully evaporated to cause crystallisation.
-
ammonia + acid ==> ammonium salt
-
e.g. (i) ammonia + hydrochloric acid ==> ammonium chloride
-
NH3(aq) + HCl(aq) ==> NH4Cl(aq)
-
or (ii) ammonia + nitric acid ==> ammonium nitrate
-
NH3(aq) + HNO3(aq) ==> NH4NO3(aq)
-
The Haber Process
The industrial manufacture of Ammonia from Nitrogen and Hydrogen gas.
Ammonia is used to make nitric acid which in turn reacts with ammonia to form ammonium nitrate, which is a fertiliser. This is a good fertiliser as plants need nitrogen to make proteins to help them grow. It is an effective fertiliser as it has nitrogen from two sources, the ammonia and the nitric acid.
Nitrogen is obtained from the air by fractional distillation.
Hydrogen is obtained from methane or naptha. The hydrogen is reacted with steam.
CH4 + 2H2O → CO2 + 4H2
The raw materials are therefore air for nitrogen, and methane and water for hydrogen.
N2(g) + 3H2(g) 2NH3(g) The forward reaction from left to right is exothermic.
Conditions
Pressure: 200 atmospheres
Temperature: 450 degrees Celcius
Catalyst: Iron
The Haber Process continued
- The reaction is reversible so there is a compromise to be made.
- Higher pressures favour the forward reaction (since there are four molecules of gas on the left hand side for every two molecules on the right).
- So the pressure is set as high as possible to give the best % yield, without making the plant too expensive to build, hence the 200 atmosphere operating pressure.
- The forward reaction is exothermic, which means that increasing the temperature will actually move the equilibrium the wrong way-away from the ammonia and towards the nitrogen and hydrogen. So the yield of ammoniua would be greater at a lower temperature.
- The trouble is, lower temperatures mean a slower rate of reaction (and so equilibrium is reached more slowly). So they increase the temperature anyway, to get a much faster rate of reaction.
- The higher temperature is an economic compromise, i.e. it is more economic to get a low yield fast, than a high yield slowly!
- The ammonia is formed as a gas, but as it cools in the condenser it liquefies and is removed. The unused hydrogen and nitrogen are recycled so nothing is wasted.
- The iron catalyst makes the reaction go faster but doesn't affect the % yield.
Energy Changes
Exothermic reactions transfer energy to the surroundings. Endothermic reactions take in energy from the surroundings.
Reversible reactions are where the products can react to remake the original reactants.
If the forward reaction is exothermic, the reverse reaction is endothermic.
Exothermic and endothermic reactions :When a chemical reaction occurs, energy is transferred to, or from, the surroundings - and there is often a temperature change. For example, when a bonfire burns it transfers heat energy to the surroundings. Objects near a bonfire become warmer. The temperature rise can be measured with a thermometer.
Exothermic reactions These are reactions that transfer energy to the surroundings. The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become hotter. The temperature increase can be detected using a thermometer.
Some examples of exothermic reactions are: burning, neutralisation reactions between acids and alkalis and the reaction between water and calcium oxide
Endothermic reactions These are reactions that take in energy from the surroundings.
The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to get colder. The temperature decrease can also be detected using a thermometer.
Some examples of endothermic reactions are: electrolysis, the reaction between ethanoic acid and sodium carbonate and the thermal decomposition of calcium carbonate in a blast furnace
Reversible Reactions
Where the products of the reaction can react with each other and convert back to the original reactants.
E.g. the thermal decomposition of ammonium chloride into ammonia and hydrogen chloride.
Dynamic Equilibrium
If reaction takes place in a close system then state of equilibrium will always be reached.
Equilibrium means that relative (%) quantities of reactants and products will reach a certain balance and stay there. (Closed system just means none of reactants/products can escape).The reactions are still taking place in both directions, but the overall effect is nil because the forward and reverse reactions cancel each other out as they are taking place at exactly the same rate in both directions.
Changing Temperature and Pressure can get you more Product
All reactions are exothermic in one direction and endothermic in the other.
If you raise the temperature, the endothermic reaction will increase to use up the extra heat.
If you reduce the temperature, the exothermic reaction will increase to give out more heat.
Most gaseous reactions have more molecules (or moles) of gas on one side than the other.
If you raise the pressure it will encourage the reaction which produces fewer molecules of gas.
If you lower the pressure it will encourage the reaction which produces more molecules of gas.
Rusting
Uses of Iron-Buiding construction, Car Manufacture, Ship Hulls
Rust only happens for iron
It occurs when iron's in contact with both oxygen and water
It is an oxidation reaction-the iron gains oxygen to form iron(|||) oxide.
Water then becomes loosely bonded to the iron(|||) oxide and the result is hydrated iron(|||) oxide-aka RUST
iron + oxygen + water → hydrated iron(|||) oxide (rust)
Methods of Prevention
- 1) Painting/Coating with plastic-ideal for small structures, can be decorative
- 2) Oiling/Greasing-when moving parts are involved like bike chains
- 3) Sacrificial Method-placing a more reactive metal with the iron. The water and oxygen react with this sacrificial metal instead of the iron.
- Zinc is often used as a sacrificial metal.
- A coating of zinc can be sprayed onto the object-known as galvanising
- Or big blocks of zinc can be bolted to the iron, eg for ship hulls or underground iron pipes
Tests-Cations
Cations are positive ions such as Na+
Method 1) Compounds of some metals burn with a characteristic colour
Clean a platinum wire loop by dipping it in some dilute HCl and holding it in a flame. Once you hold the wire in the flame and it burns without any colour, you can dip it in the sample you want to test then put it back in the flame to see what ion is present.
Lithium-Li+ Burns with a crimson-red flame
Sodium-Na+ Burns with a yellow-orange flame
Potassium-K+ burns with a lilac flame
Calcium-Ca2+ burns with a brick-red flame
Method 2) Some metals form a coloured precipitate with NaOH
Many metal hydroxides are insoluble and precipitate out of solution when formed. You add a few drops of NaOH solution to a mystery compound in a test tube, trying to form an insoluble hydroxide. If you get a coloured one, you can tell which metal was in the compound.
Copper(||)-Cu2+ Blue Cu2+(aq)+2OH-(aq) → Cu(OH)2(s)
Iron(||)-Fe2+ Sludgy Green Fe2+(aq)+2OH-(aq) → Fe(OH)2(s)
Iron(|||)Fe3+ Reddish brown Fe3+(aq)+2OH-(aq) → Fe(OH)3(s)
Tests-Gases and Water
1) Ammonia-Using a damp red piece of litmus paper. If there's ammonia present the paper will turn blue. (It has to be damp so the ammonia gas can dissolve and make the colour change).
Also to see if something has NH4+ ions, you can add some sodium hydroxide to your substance and see if ammonia is present which means ammonium ions are present.
2) Chlorine-Bleaches damp litmus paper, turning it white.
3) Oxygen-Relights a glowing splint
4) Carbon Dioxide-Turns limewater cloudy
5) Hydrogen-Makes a squeaky pop with a lighted splint
6) Water-Add a few drops of your solution to white anhydrous copper sulfate, and if the crystals go blue, it will be hydrated copper sulfate and that will mean water is present.
To check water is pure-
The boiling point is-100 degrees Celcius
The freezing point is-0 degrees Celcius
Tests-Anions
Anions are negative ions such as Cl-
Carbonates
To test for these, add dilute hydrochloric acid to your sample. If carbonates are present then carbon dioxide will be released and you can test for that using limewater.
Sulfates
- Add dilute HCl (to get rid of any traces of carbonate or sulfite ions before the test, as both of those would produce a precipitate and confuse the results).
- Then you add barium chloride solution BaCl2
- A white precipitate of barium sulfate means the original compound was a sulfate.
Halides
- To test for chloride, bromide or iodide ions, add dilute nitric acid, HNO3 (again added to get rid of carbonate and sulfite ions)
- Then add silver nitrate solution AgNO3
- A chloride ion gives a white precipitate of silver chloride
A bromide ion gives a cream precipitate of silver bromide
An iodide ion gives a yellow precipitate of silver iodide
Alkanes
- A homologous family (series) of chemicals
- Hydrocarbons
- Saturated-no double bonds
Formula: CnH2n+2
The amount of carbons names the alkane
1: Methane CH4
2: Ethane C2H6
3: Propane C3H8
4: Butane C4H10
5: Pentane C5H12
6: Hexane C6H14
Alkanes are joined together by covalent bonds
Combustion of Alkanes
- Alkanes are all fuels
- All burn with oxygen
- These are combustion reactions
- They form CO2 and H2O
Complete combustion occurs when there is sufficient or excess oxygen present
alkane + oxygen → carbon dioxide + water (+energy)
When combustion is complete, the gas burns with a clean blue flame
Incomplete combustion occurs when there is not sufficient oxygen
It is not safe, as it also produces carbon and carbon monoxide, and CO is a poisonous gas.
alkane + oxygen → carbon + carbon monoxide + carbon dioxide + water (+energy)
When combustion is incomplete, the flame will be smoky-yellow, and less energy will be produced.
Haloalkanes
Halogens react with alkanes in the presence of UV light to form Haloalkanes
In these reactions a hydrogen atom from the alkane is substituted with chlorine or bromine so this is a substitution reaction.
E.g. methane + bromine → bromomethane + hydrogen bromide
CH4+ Br2 → CH3Br + HBr
Halogens react with alkenes to form Haloalkanes
It is called an addition reaction, because the double bond splits up and two halogen atoms are added, one to each carbon.
E.g. ethene + bromine → dibromoethene
C2H4 + Br2 → C2H4Br2
It's called dibromoethane because it has two bromine atoms
How to tell Alkanes and Alkenes apart
Shake the hydrocarbon with orange bromine water
If it goes colourless, it is an alkene, as it has reacted with the double bond to form a colourless dibromoalkane.
Bromine water will stay orange with an alkane because it has no double bond.
Alkenes and Isomers
- Another homologous (same structure) family of hydrocarbons
- Unsaturated-double bonds
- Formula: CnH2n
- They can only ever have one double bond
- Carbon can only make 4 bonds
- They are unsaturated as the double bond can open up allowing the carbon atoms to bond with other atoms
Isomers
Have the same molecular formulas but different structures
E.g. Butene C4H8 has two different structures
Synthetic Ethanol
Ethanol can be produced from Ethene and Steam
Ethene is produced from crude oil (cracking)
Ethene C2H4 will react with steam H2O to make ethanol.
C2H4 + H2O → C2H5OH
Temperature: 300 degrees Celcius
Pressure: 60-70 atmospheres
Catalyst: Phosphoric acid
Continuous process
No waste products
Less land needed
Low labour costs
Very fast
Pure
Very Concentrated
High amounts of energy needed
Uses oil which is non-renewable
Expensive equipment
Yeast Fermentation
The raw material for fermentation is sugar aka glucose.
This is converted into ethanol using yeast.
C6H12O6 2C2H5OH + 2CO2
Temperature: 30 degrees Celcius
Pressure: Normal
Catalyst: Enzymes
Stop and start (Batch) process
Sugar is renewable
Simpler and cheaper equipment needed
Low amounts of energy needed
High labour costs
Very slow
Very impure
Carbon Dioxide released as waste product
Large areas of land needed
Dehydration of Ethanol
You can turn ethanol back into ethene
Remove water from the ethanol in a dehydration reaction
C2H5OH → C2H4 + H2O
Ethanol vapour is passed over a hot catalyst of aluminium oxide Al2O3- the catalyst provides a large surface area for the reaction
Fractional Distillation of Crude Oil
Crude oil is a mixture of hydrocarbons that can be used to produced energy
The different compounds in crude oil can be separated.
1) The oil is heated into most of it turns into gas.
2) The gases enter a fractionating column.
3) In the column there is a temperature gradient (hot at bottom, cooler at top). When substances reach part of the column when the temperature is lower than their boiling point, they condense, draining out at different points into different fractions.
- At the beginning of the process, the remaining liquid bit, bitumen is drained off at the bottom.
- Shorter hydrocarbons have lower boiling points. They condense and drain out much later on near to the top where it is cooler.
- Long hydrocarbons have higher boiling points. They condense and drain out earlier near the bottom.
- Bubble caps in the fractionating column stop the separated liquids from running back down the column and remixing.
Gasoline is a short hydrocarbon used to fuel cars
Diesel is a longer chain hydrocarbon used for diesel engines.
Cracking Hydrocarbons
- Long chain hydrocarbons are viscous (thick and gloopy) and have high boiling points.
- Short chain hydrocarbons have lower boiling points and are thinner and paler in colour.
- Long chain hydrocarbons aren't really useful. Demand for short is higher.
- Cracking is splitting them up into shorter more useful ones using thermal decomposition.
long chain alkane → short chain alkane + alkene
e.g. dodecane (found in paraffin) → octane + ethene
Temperature: 600-700 degrees Celcius
Catalyst: Silica or alumina
Cracking Paraffin in the Lab
1) Heat the paraffin. After a few seconds move the bunsen burner to heat the silica or alumina catalyst. Alternate between paraffin and catalyst until the paraffin vapourises and the catalyst glows red.
2) The heated paraffin vapour cracks as it passes over the heaeted catalyst.
3) Small alkanes collect at the end of the boiling tube, whilst alkene gases travel down the delivery tube
4) The alkenes are then collected through water using a gas jar.
Polymers
Hydrocarbon-made up of hydrogen and carbon molecules only
Monomer-a small molecule that can join to other small molecules
Polymer-when lots of monomers join together
Polymerisation-the process by which monomers join together to form a polymer
Addition Polymers
- Monomers with a double bond
- The double bond breaks
- Lots of monomers then join together to make a polymer e.g ethene to polyethene
- Happens under high pressure and with a catalyst
Repeat Units
Polymers 2.0
Condensation Polymerisation
- Usually involves two different types of monomer
- The monomers react together and bonds form between them, making polymer chains
- It produces the polymer plus another small molecule (e.g. water)
- Nylon is an example
Uses of Polymers
Different polymers have different physical properties which makes them suitable for different uses.
1) Polyethene is a light, stretchable polymer. This makes it ideal for packaging such as plastic bags, bottles and containers.
2) Polypropene is a very tough polymer, but relatively flexible and easy to heat. Used to make kettles, carpets.
3) Polychloroethene is used to make clothes and pipes and for insulating electrical cables
Polymers 3.0
Modifying Polymers Chain length-increasing the chain length makes the material stronger and stiffer, and the melting point gets higher. Plasticisers-a chemical added to a polymer to increase a polymer's flexibility. The plasticiser gets between the polymer chains and keeps them further apart which reduces the forces of attraction between them and makes the material more flexible. Cross links-Chemical bonds can be added between the chains to make the material tougher and less flexible. Problems with Polymers
- Most polymers are inert-they don't react easily.
- Polymers are non-biodegradable, so bacteria and other organisms cannot break them down. Well it takes them a really long time. This is because the bonds are strong and hard to break.
- So they end up just sitting in rubbish dumps.
Ionic Bonding
Atoms lose or gain electrons to form charged particles (ions) which are then strongly attracted to one another (because of the attraction of the opposite charges + and -). This strong attraction is known as electrostatic attraction-it gives ionic compounds their high melting and boiling points.
A shell with just one electron is well keen to get rid of it.
A shell with a near full shell is well keen to get that extra electron.
They become ions which are attracted to each other because of the opposite charges.
E.g. sodium and chlorine.
The sodium atom gives up its outer electron and becomes an Na+ atom.
The chlorine atom picks up the spare electron and becomes a Cl- atom.
Groups 1&2 and 6&7 are most likely to form ions.
Group 1&2 elements are metals and lost electrons to form +ve ions (cations).
Group 6&7 elements are non-metals and gain electrons to form -ve ions (anions).
When any of these cations meet up with any of these anions, they form ionic compounds.
Dot and Cross Diagrams of Ionic Bonding
Giant Ionic Structures
- Ionic compounds always have giant ionic structures. The ions are held together in a closely packed 3D lattice arrangement by the attraction between oppositely charged ions.
- The electrostatic attraction between oppositely charged ions is very strong. Because a lot of energy is needed to overcome the strong attraction, this means that ionic compounds have high melting and boiling points.
- The charges on the ions in the lattice also affect the strength of the ionic bonding. A lattice of 2+ and 2- ions is held together by stronger forces of attraction than a lattic of 1+ and 1- ions. This means lattices made up of higher charge ions have higher melting/boiling points.
Sodium Chloride has a typical ionic structure.
Covalent Bonding
- Shared pair of electrons.
- Each atoms feels they have full outer shell and are happy.
- Strong attraction between the shared electrons and the nuclei of the atoms involved.
Dot and Cross Diagrams
Covalent Substances
Substances containing covalent bonds can be simple molecules or giant structures.
Simple Moleculer Substances
1) The atoms within a molecule are held together by very strong covalent bonds
2) By comparison, the forces of attraction between the molecules are very weak
3) The result of these weak intermollecular forces is that the melting and boiling points are very low because the moecules are easily parted from one another.
4) Most molecular substances are gases or liquids at room temperature.
5) You can tell a molecular from its physical state, which is mushy.
6) They are also non-conductive as they do not have any free electrons or an overall electric charge.
Giant Covalent Substances
1) Similar except there are no charged ions.
2) All atoms are bonded to each other by strong by covalent bonds.
3) Lots of these bonds meaning it takes lots of energy to break them so they have very high melting and boiling points.
4) Don't conduct electricity (except for graphite).
5) Usually insoluble in water
Diamond
DIAMOND
In diamond, each carbon atom forms four covalent bonds in a very rigid giant covalent structure. This structures makes diamond the hardest natural substance, so it's used for drill tips and cutting tools.
Graphite
GRAPHITE
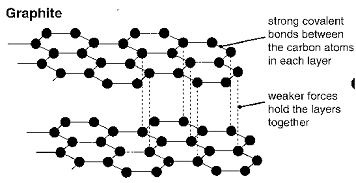
In graphite, each carbon atom only forms three covalent bonds, creating layers which are free to slide over each other. This makes graphite useful as a lubricant. It also leaves free electrons, so graphite is the only non-metal which is a good conductor of electricity.
Isotopes
Different atomic forms of the same element, which have the same number of protons, but different numbers of neutrons
Relative Atomic Mass Takes All Stable Isotopes Into Account
It's the average mass of all the isotopes of an element. It has to allow for the relative mass of each isotope and its relative abundance.
Relative abundance just means how much there is of each isotope compared to the total amount of the element in the world. Can be a ratio, fraction or percentage.
E.g Chlorine
Relative mass of isotope 35 37
Relative abundance 3 1
1) Multiply the mass of each isotope by its relative abundance
2) Add those together
3) Divide by the sum of the relative abundance
(35x3) + (37x1) / 3+1 = 35.5
Electrical Conductivity
ELECTRIC CURRENT IS A FLOW OF ELECTRONS OR IONS
1) Electrons have a negative charge. Ions can either have a +ve or -ve charge.
2) Electrons and ions can act as charge carriers-they can move charge around a system to create a flow of electricity.
IONIC COMPOUNDS ONLY CONDUCT ELECTRICITY WHEN MOLTEN OR IN SOLUTION
1) ICs are made up of a lattice of +ve and -ve ions.
2) Solid ICs don't conduct electricity because the ions aren't free to move around.
3) When an IC is dissolved the ions separate and are free to move in the solution. They'll carry electric current and so conduct electricity.
COVALENT COMPOUNDS DON'T CONDUCT ELECTRICITY
1) CCs don't contain ions because they make bonds by sharing electrons, so they don't have any charge carriers that are free to move so can't carry an electric current.
Metals
METALS ARE HELD TOGETHER BY METALLIC BONDING
1) Metals have a giant structure of positive ions surround by a sea of delocalised (free electrons).
2) The attraction between the +ve ions and electrons is called metallic bonding.
3) It is this metallic bonding that gives metals their properties
METALS ARE GOOD CONDUCTORS OF ELECTRICITY AND HEAT
The free electrons carry electrical current and heat energy through the material, so metals are good conductors of electricity and heat.
MOST METALS ARE MALLEABLE
The layers of atoms in a metal can slide over each other, making metals malleable-this means that they can be hammered or rolled into flat sheets.
Electrolysis
Used to make new substances
-Electrolysis is when if you pass an electric current through an ionic substance that's molten or in solution it breaks down into new substances.
-It requires a liquid to conduct the electricity called the electrolyte.
-Electrolytes are made by melting or dissolving ionic compounds.
-It's the free ions that conduct the electricity.
-For the circuit to be complete, there has to be a flow of electrons. Electrons are taken away from ions at the postive electrode (anode) and given to ions at the negative electrode (cathode).
-As ions gain or lose electrons they become atoms or molecules.
Electrolytes are liquids that conduct electricity
-When you place an electricity probe in an electrolyte, current flows through the circuit so you can measure its conductivity
-When you place a conductivity probe in a non-electrolyte, no current flows so you'll get a reading of zero conductivity.
-Another way of determining whether a substance is electrolyte or not is to set up an electrolytic cell.
-If the substance undergoes electrolysis, then it is an electrolyte.
Electrolysis of Ionic Compounds Example
- Molten ICs can be electrolysed because they have freely moving ions.
- They are usually broken up into their elements.
- An example of this is the electrolysis of molten lead bromide.
- The +ve Pb2+ ions are attracted to the -ve cathode. At the cathode a lead ion accepts two electrons to become a lead atom. The molten lead that forms will sink to the bottom.
Pb2+(l) + 2e- → Pb(l) - The -ve Br- ions are attracted to the +ve anode. At the anode two bromide ions lose one electron each and become a bromine molecule. Brown bromine gas forms at the top of the anode.
2Br-(l) → Br2(g) + 2e- - The electrodes are made from an inert material so they don't take part in the reaction. Common ones are carbon (graphite), iron and platinum.
Electrolysis of Aqueous Solutions
An aqueous solution of a compound is a mixture of two electrolytes, it's a compound dissolved in water really (so water is the solvent).
Therefore in aqueous solutions, as well as the ions from the ionic compound, there will be hydrogen ions and hydroxide ions from the water.
At the cathode, if H+ ions and metal ions are present, hydrogen gas will be produced if the metal ions are more reactive than the H+ ions. If the metal ions are less reactive than the H+ ions, a solid layer of pure metal will be produced.
At the anode if OH- and halide ions (Cl-,Br-,I-) are present, then molecules of chlorine, bromine or iodine will be formed. If no halide ions are present then oxygen will be formed.
Electrolysis of Brine (Sodium Chloride Solution)
- Electrolysed using a diaphragm cell.
- Produces three useful products.
- Hydrogen gas is given off at the cathode-two H+ ions accept two electrons to become one hydrogen molecule.
2H+ + 2e– → H2 (reduction) - Chlorine gas is given off at the anode-two Cl- ions lose their electrons and become one chlorine molecule.
2Cl– – 2e– → Cl2 (oxidation) - The Na+ ions stay in the solution and the OH- ions from the water are also left behind. This means that sodium hydroxide (NaOH) is left in the solution.
Uses
Chlorine: Sterilise water supplies (chlorination). To make bleach and HCl.
Hydrogen: Used in haber process, and to change oils→ fats for making margarine
Sodium Hydroxide: Chemical industry making soap, bleach and paper pulp.
Collision Theory
- Collision theory is that a chemical reaction can only occur between particles when they collide.
- Particles can be atoms, ions or molecules.
- There is a minimum amount of energy which colliding particles need in order to react.
- If the particles have less than this minimum energy then they just bounce off each other and no reaction occurs.
- This minimum energy is called activation energy.
- The faster the particles are going the more energy they have.
- Fast moving particles are more likely to react when they collide.
- You can make particles move faster by heating them only
Rate of Reaction
HIGHER TEMPERATURE
When the temperature is increased the particles have more energy and move quickern which means they're going to collide more frequently.
HIGHER CONCENTRATION (or PRESSURE)
If a solution is made more concentrated it means more particles of reactant knocking about between the water molecules which makes collsions between important particles more likely.
In a gas, increasing the pressure means the particles are more squashed up together so they are going to collide more frequently.
As a reaction progresses there are fewer and fewer reactant particles so they collide less frequently and the reaction rate slows down.
LARGER SURFACE AREA
If one of the reactants is a solid then breaking it up into smaller pieces will increase the surace area. This means the particles around it in the solution will havem ore area to work on, so there'll be useful collisions more often.
CATALYSTS
A solid catalyst works by giving the reacting particles a surface to stick to. They increase the no. of successful collisions by lowering the activation energy.
Measuring Rates of Reaction
Rate of reaction = Amount of reactant used or amount of product formed / time
Precipitation
1) When product of the reaction is a precipitate which clouds solution.
2) Observe a marker through solution and measure how long it takes for it to disappear
3) Quicker it disappears, quicker the reaction
4) Only works for solutions which are initially quite see-through
5) Result is subjective, people argue over when mark disappears
Change in Mass (Usually Gas Given Off)
1) Measuring speed of reaction that produces a gas can be carried out on mass balance
2) As gas is released, mass disappearing is easily measured on the balance
3) Quicker the reading on the balance drops, faster the reaction
4) When mass stops changing, reaction's finished.
5) Rate of reaction graphs easy to plot using this method's results
6) Most accurate as mass balance is very accurate, but releases gas straight into room.
The Volume of Gas Given Off
1) Gas syringe measures
2) The more gas given off during given time interval, faster the reaction
3) When gas stops being produced, reaction's finished
4) A graph of gas volume against time elapsed could be plotted
5) Gas syringes are quite accurate (nearest mm) but reaction vigorous so plunger could easily be blown off the end
RoR Experiment: HCl and Marble Chips
1) Measure volume of carbon dioxide gas evolved with gas syringe and take readings at regular intervals
2) Make table of readings and plot them as graph. You choose regular time intervals so time is the independent variable and volume is the dependent variable.
3) Repeat the experiment with exactly the same volume of acid, and exactly the same mass of marble chips, but with the marble more crunced up (surface area)
The rate is not a constant throughout the reaction - it changes!
The reaction is fastest at start, becoming slower as the reaction proceeds.
From the graph, the fastest part of the reaction is shown by the steepest curve.
The curve on the graph goes flat when the reaction is complete. This is because, as time goes on the volume of the gas evolved does not change.
An increase in surface area causes more frequent collisions so the rate of reaction is faster.
If you increased the amount of chips, the extra surface area gives a quicker reaction and there is also more gas evolved overall as long as the acid is in excess.
RoR Experiment: Magnesium and Dilute HCl
1) Good for measuring the effects of increased concentration
2) Gives off hydrogen gas, which we can measure with a mass balance
3) In this experiment, time is the independent variable and mass loss is the depenedent variable
- Take readings of mass at regular time intervals
- Put results in a table and work out loss in mass for each reading. Plot a graph
- Repeat with more concentrated acid solutions but always the same amount of magnesium
- The volume of acid must always be kept the same too, only the concentration is increased
- A higher concentration gives a steeper graph, with the reaction finishing much quicker
RoR Experiment: Sodium Thiosulfate and HCl
1) These two chemicals are both clear solutions
2) They react together to form a yellow precipitate of sulfur
3) The experiment involves watching a black mark disappear through the cloudy sulfur and timing how long it takes to go (dependent)
4) The reaction can be repeated for solutions at different temperatures (independent) In practice, that's quite hard to do accurately and safely, so use a water bath to get each to the right temperature before you mix them
5) The depth of liquid must be kept the same each time (control)
6) The results will show that the higher the temperature, the quicker the reaction and therefore the less time it takes for the mark to disappear.
RoR Experiment: The Decomposition of Hydrogen Pero
2H2O2 (aq) → 2H2O (l) + O2 (g)
Good reaction for showing effects of different catalysts (independent) on rate of reaction.
1)The decomposition is normally quite slow, but manganese(IV) oxide catalyst speeds it up loads.
2) Oxygen is given off (dependent), which provides an ideal way to measure the rate of reaction using the gas syringe method.
3) Plot graphs for different catalysts.
4) Better catalysts give a quicker reaction which is shown by a steeper graph which levels off quickly.
5) The graphs will look the same if you do it with temperature or concentration being the independent variable.
Energy Transfer in Reactions
In a chemical reaction, energy can be transferred to or from the surroundings and it's all about making and breaking bonds.
Energy must always be supplied to make bonds.
1) During a chemical reaction, old bonds are broken and new bonds are formed.
2) Energy must be supplied to break existing bonds, so bond breaking is an endothermic process.
3) Energy's released when new bonds are formed, so bond formation is an exothermic process.
In an Endothermic Reaction, Energy is Taken In
In an endothermic reaction, the amount of energy required to break old bonds is greater than energy released when new bonds are formed.
An endothermic reaction is one which takes in energy from its surroundings, usually in the form of heat, and usually shown by a fall in temperature.
In an Exothermic Reaction, Energy is Given Out
In an exothermic reaction, the energy released in bond formation is greater than the energy used in breaking old bonds.
An exothermic reaction is one which gives out energy to its surroundings, usually in the form of heat, and usually shown by a rise in temperature.
Enthalpy Change
The overall change in energy in a reaction is called the ENTHALPY change. It has the symbol ΔH.
1) The units of ΔH are kJ/mol, so it's the amount of energy in kilojoules per mole of reactant.
- If the reaction is endothermic, the value is positive because the reaction takes in energy.
- If the reaction is exothermic, the value is negative because the reaction's giving out energy.
Endothermic Energy Level Diagram
The products are at a higher energy than the reactants, so ΔH is +ve.
Exothermic Energy Level Diagram
The products are at a lower energy than the reactants.
The different in height represents the energy given out in the reaction (per mole).
ΔH is -ve here.
The initial rise in the line represents the energy needed to break the old bonds. This is the activation energy.
Activation Energy Lowered By Catalysts
The activation energy represents the minimum energy needed by reacting particles for the reaction to occur.
A catalyst makes reactions happen faster by providing an alternative pathway with a lower activation energy.
This is represented by the lower curve on an energy level diagram, which shows that less initial energy is needed for the reaction to begin.
The overall energy change for the reaction ΔH remains the same though.
Group 7-The Halogens
As you go down the group, (as the atomic number increases), the elements have a darker colour and a higher boiling point. This is why they go from gases at the top of the group to solids at the bottom.
The higher up Group 7 an element is, the more reactive it is. This is as the shell with the missing electron is nearer to the nucleus, so the pull from the positive nucleus is greater.
Dissociation
Halogens can combine with hydrogen to form hydrogen halides e.g. HCl.
- When hydrogen chloride is dissolved in water, the HCl molecules split up into H+ ions and Cl- ions, this is dissociation.
- The solution that is formed is hydrochloric acid.
- Hydrochloric acid is an acidic solution because it contains H+ ions.
- When HCl is dissolved in an organic solvent like methylbenzee, it doesn't dissociate into H+ and Cl- ions.
- This means there are no H+ ions so it's not acidic.
If there is water on the paper or in the bottle, this could make the HCl dissociate and behave like an acid again.
Group 1-The Alkali Metals
All have one electron in outer shell, so are very reactive.
They all react in a similar way in water, vigorously, and producing a metal hydroxide. This is alkaline hence the name. The reaction also produces hydrogen which is why you can see fizzing.
e.g. Sodium + Water → Sodium Hydroxide + Hydrogen
Group 1 Elements become more reactive down the group-as the atomic number increases
Lithium + water: Moves slowly around the surface, fizzing until it disappeasrs. 30 secs
Sodium + water: Fizzes rapidly and moves quickly around the surface, and may ignite. 20 secs
Potassium + water: Reacts vigorously, burns with lilac flame, sometimes explodes. 5 secs.
The must be stored in oil or they react with the oxygen in the air.
Atoms lose electrons more easily down the group
All have 1 electron in outer shell
As you go down group 1, the outermost electron is in a shell that's further from the nucleus
This means the attraction between the outermost electron and the nucleus becomes less
So as you go down group 1, the atoms get bigger, the outer electron is more easily lost, and the metals are more reactive.
Group 0-The Noble Gases
Including: Helium, neon, argon etc
They are inert, meaning they don't react with much at all
They have a full shell of outer electrons, so they aren't desperate to give up or gain electrons.
Metal Ores
- Most metals react with other elements to form compounds, which can be found naturally in the Earth's crust. If a compound contains enough of the metal to make it worthwhile extracting, the compound is called a metal ore. There are limited amounts of metal ores, they are finite resources.
- The more reactive a metal is, the harder it is to extract it from a compound.
Metals often have to be separated from the Oxides
Lots of common metals like iron and aluminium react with oxygen to form oxides.
These oxides are often ores that the metals need to be extracted from.
A reaction that separates a metal from the oxygen is called a reduction reaction.
In a reduction reaction, the substance that reduces the metal (and is oxidised) is called the reducing agent.
The most common type of reduction reaction uses carbon as a reducing agent.
But carbon can't be used for all metals.
Only metals that are less reactive than carbon can be extracted by a reduction reaction with carbon, e.g. zinc, iron, tin, copper, lead, platinum, silver and gold.
Metals that are more reactive than carbon have to be extracted using electrolysis.
Extracting Aluminium
Electrolysis removes aluminium from its ore
The main ore is bauxite, and after mining and purifying, a white powder is left
This is pure aluminium oxide Al₂O₃
Al₂O₃has a very high boiling point of over 2000°C, so melting it would be very expensive.
Instead the aluminium oxide is dissolved in molten cryolite (less common aluminium ore).
This brings the temp. to around 900°C, which makes it cheaper and easier.
The electrodes are made of graphite, a good conductor of electricity.
Turning the Ions into the Atoms you want
Molten aluminium oxide contains free ions so it'll conduct electricity.
The +ve Al3+ ions are attracted to the -ve cathode where they pick up electrons and turn into aluminium atoms, then they sink to the bottom where they can be tapped off.
Al3+ + 3e- → Al <Reduction (gain of electrons)
The -ve O2- ions are attracted to the +ve anode where they lose electrons. The oxygen atoms will then react together to form O2, or with the carbon anode as well to form CO2.
2O2- → O2 + 4e- <Oxidation (Loss of electrons)
As the +ve carbon electrode is constantly getting worn down by reacting with oxygen, it needs replacing often.
Electrolysis is expensive
Uses a lot of electricity, energy is needed to heat the electrolyte and the anode needs replacing.
However overall it is relatively cheap as it is widely used.
Extracting Iron
Iron is extracted from it's ore haematite (Fe2O3, Iron(|||)Oxide) by reduction in a blast furnace.
Raw materials are iron ore, coke and limestone
Coke is pure carbon. This is to reduce the iron oxide to iron metal.
Limestone takes away the impurities in the form of ****.
In the furnace
Hot air is blasted into the furnace, making coke burn much faster than normal. this raises the temp. to about 1500°C.
1)The coke burns and produces carbon dioxide. C + O2 → CO2
2) The CO2 then reacts with unburnt coke to form CO. CO2 + C → 2CO
3) The CO then reduces the the iron ore to iron. 3CO + Fe2O3 → 3CO2 + 2Fe
The iron is molten at this temp. and very dense, so it runs straight to the bottom of the furnace where it's tapped off.
Removing the Impurities
Main impurity is sand (silicon dioxide). This is still solid even at 1500°C and would tend to stay mixed in with the iron. The limestone removes it.
1) The limestone is decomposed by the heat into calcium oxide and CO2.
CaCO3 + CaO → CO2
2) The calcium oxide then reacts with sand to form calcium silicate, or **** which is molten and can be tapped off.
CaO + SiO2 → CaSiO3 (molten ****).
The cooled **** is solid and used for road-building and fertiliser.
Iron and Aluminium Shared Properties
1) Both dense and lustrous
2) Have high melting points
3) High tensile strength (strong and hard to break)
4) Malleable (hammered into diff. shape)
5) Good conductors of electricity and heat energy
Uses of Iron
Adding other materials to the iron can change its properties, making it rather useful.
1) Wrought iron is almost completely pure iron. Malleable so used to make gates and railings.
2) You can then mix iron with other elements to make alloys.
Cast iron is a mixture of iron, carbon and silicon. It's very hard, but brittle. Cast Iron is used for manhole covers and some cooking pans.
Steel is an alloy made of iron, carbon and usually some other metals. Steel has more useful properties than iron, it's harder, but still malleable. It is good or making car bodies and girders.
The problem with iron is that it rusts.
Stainless steel is an alloy made of iron and chromium that doesn't rust. It's used for cutlery and cooking pans.
Uses of Aluminium
Typical metal. Doesn't corrode easily.
Reacts very quickly with oxygen in the air to form aluminium oxide. A protective layer of aluminium oxide sticks firmly to the aluminium beow and stops further reaction taking place.
Because it doesn't corrode, it's useful for products that come in contact with water e.g. drink cans.
Aluminium is less dense than iron, so it is lighter
This makes it useful when weight of metal is important, like in aeroplanes.


Comments
No comments have yet been made