Chemistry
- Created by: ELIRAWLI
- Created on: 02-11-16 10:22
Volume of Gas
Standard temperature and pressure (STP) is 0*C and 1 atmosphere (atm).
At STP, 1 mol of gas ocupies a volume of 22.4L.This is known as the MOLAR VOLUME of a gas.
Volume of gas = number of moled x 22.4
V = n x 22.4
n = V/22.4
Properties of Gas
Gases all have similar properties:
1. Low density fompared with liquids or solids
2. They spread to fill the container in which they are placed
3. They exert pressure in all directions
4. They can diffuse (mix) easily and quickly
5. They are easily compressed
Kinetic Molecular Theory
The properties of gases can be explained through the kinetic molecular theory. The particles in a gas are usually molecules or atoms.
According to this theory:
1. Gases are made up of particles moving with rapid, constant random motion.
2. The higher the temperature, the faster the particles move. They have increased kinetic energy.
3. The forces of attraction and repulsion between teh gas particles are practically zero.
4. The particles are very far apart. The volume of the particles is very small compared with the volume that the gas occupies
5. Particles collide withe ach other and the walls of the container exerting pressure. The collisions with each other are perfectly elastic. This means that no kinetic energy is lost when they collide with each other.
Kinetic Molecular Theory Questions
Use the kinetic molecular theory of gases to explain the following:
1. Gases can be compressed
Gases can easily be compressed becuase the as particles are far apart
2. Gases diffuse easily
Gases diffuse easily as they are in a constant random motion and move very rapidly. They will keep moving until they hit something
3. Gases fill the container
Gases fill the container because they are moving in a constant random motion, rapidly and they will keep moving and spreading out until they hit something.
4. Gases are less dense than liquids or solids
Gases have the ability to move past freely, meaning they are more spread out than liquids or solids, thus much less dense.
Gas Pressure
The pressure of a gas is due to the gas particles colliding with the walls of the container.
A barometer is a device that is used to measure atmospheric pressure.
Pressure is measured in a number of units. The SI unit of pressure is the pascal (Pa). Older units of pressure that are still in use are millimetres of mercury (mm Hg) and atmospheres (atm).
1 atm = 760 mm Hg = 101.3 kPa
Converting Units (Gas pressure)
kPa --> atm divide by 101.3
atm --> kPa multiply by 101.3
mm Hhg --> atm divide by 760
atm --> mm Hg multiply by 760
Boyle's Law
Increasinf the pressure on a sample of gas will lead to a decrease in volume (keeping it the same temperature).
At constant temperature:
P(1)V(1) = P(2)V(2)
Note: Tthis only applies to an "Ideal" gas.
An ideal gas is one that follows the 5 basic assumptions of the kinetic molecular theory.
Charle's Law
Changing the temperature of a gas directly changes the volume occupied. I.e. lower temp means smaller volume, higher temp means larger volume.
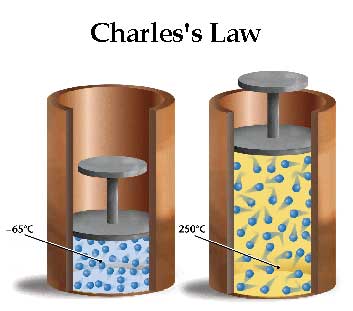
For a a fixed amount of gas at a constant pressure:
V(1)/T(1) = V(2)/T(2)
Note: Temperature must be in kelvin
Note: To convet from celsius to kelvin, must add 273.
Gay-Lussac's Law
If the volume of a gas is ket constant, as the temperatureof the gas increases, the pressure inreases.
At constant volume:
P(1)/T(1) = P(2)/T(2)
Note: temperature must be in Kelvin.
The Combined Gas Law
Tthe combined gas law combines Boyle's, Charle's and Gay-Lussac's laws together.
The combined gas law allows you to do calculation when the amount (moles) of gas is constant:
P(1)V(1) = P(2)V(2)
T(1) T(2)
Manipulating the Formula
The combines gas law cna be altered if pressure, volume or temperature is kept constant.
Re write the formula when:
a) Pressure is constant - V(1)/T(1) = V(2)/T(2) (Charle's Law)
b) Volume is constant - P(1)/T(1) = P(2)/T(2) (Gay-Lussac's Law)
c) Temperature is constant - P(1)V(1) = P(2)V(2) (Boyle's Law)
Ideal Vs Real Gases
An ideal gas is a gas that obeys all the gas laws perfectly at a particular temperature and pressure. No gas behaves ideally, however, at all temperatures and pressures. Real gases show large departures from ideality, particularly at high pressures and low temperatures.
- The kinetic theory assumes no attraction between particles and negligible particle volume
- Ideal gases obey the gas laws at specific temperatures and pressures
- No gases obey these laws at all temperatures and pressure
Real gases will not follow the gas laws at very high pressure or very low temperatures. This is because the size of the gas particles become important at high pressures and low temperatures. Tha gas particles become closer together and take up more space then predicted. Therefore forces between them exist. E.g. They become liquids or solids.
The Ideal Gas Law
For an ideal gas (a gas tat obeys the kinetic molecular theory):
PV = nRT
Where:
P = pressure V = volume n = the number of moles R = the universal gas constant T = temperature in kelvin
The value of R depends on the units used for pressure:
R=62.4 if pressure is mm Hg
R=0.082 is pressure is atm
R=8.31 if pressure is kPa
Dalton's Law
The total pressure in a gas mixture is the sum of the partial pressures of each individual gas.
P(Total) = P(1) + P(2) + P(3) + ...
Dalton's Law in the Human Body
Dalton's law can help determine if we can survive in a particular environment.
Humans need a partial pressure of about 11kPa of 0(2) to survive but cannot exceed 160kPa of O(2).
The partial pressure of gases in the atmosphere is as follows
N(s) 78% O(2) 21% Ar 0.9% CO(2) 0.04% other gases 0.06%
How can we dive safely to deeper deaths?
If we reduce the O(2) and remove the N(2) from the air and replace it with a non-toxic unreactive gas (helium) then we can dive deeper.
At high pressures increased amounts of nitrogen enters the blood. When a diver ascends rapidly the nitrogen expands caussing bubbles in the bloodstream (the "bends").
Helium is less soluble in wter than nitrogen and does not enter the bloodstream. This is because it diffuses at a faster rate.
Graham's Law
Diffusion is the tendency of gases to spread out. Effusion is when a gas escapes through a tiny hole in its container (E.g. Balloons go flat)
Graham's law of effusion shows that lighter gases effuse faster than heavier gases.
Rate(1)/Rate(2) = the square root of M(2)/M(1)
Graphing of Gas Laws
Charles Law
Boyle's Law
Enthalpy and Thermochemical Equations
Enthalpy (H) is the heat content of a system.
The change in enthalpy /\ H is given by:
/\ H = H (products) - H (reactants)
Exothermic Reactions
- Heat is released too the surroundings
- /\ H is negative
- feel hot
Example:
The combustion of methane:
CH(4) + 2O(2) -----> 2H(2)O + CO(2) ; /\ H = -890.4 kJ/mol
This means that for every 1 mole of CH(4) this is combusted, 890.4kJ of energy is released.
Endothermic Reactions
Energy Content in Foods
Aim: To calculate the energy vontent of various foods by measuring the heat released when the foods are burnt.
ETC...
breathing and Respiration
Respiration is a chemical reaction that occurs in our cells to provide energy. During aerobic (with oxygen) respiration, our bodies use glucose to obtain energy.
Enzymes
Enzymes are macromolecular biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The optimal temperature is about body temperature (37*C).
Reversible Reactions
A reversible reaction is one in which the conversion of reactant to products, and teh conversion of products to reactants occur simultaneously.
When teh rates of the foward and reverse reactoin are equal, a sate of balance called chemical equilibrium has been reached.
Examples: Demonstration with fish tanks
2SO(2)(g) + O(2) <---> 2SO(3)(g)
When SO(2) and 0(2) are mixed in a sealed container the reverse reaction is low at first. The revere reaction then increases until the rate of foward reaction equals teh rate of reverse reaction. Equilibrium has then been reached
Le Chatelier's Principle
Le Chatelier's Principle states that if a change is applied to a system at equilibrium, the system reacts in such a way as to counteract the change, in order to return to equilibrium.
When a system at equilibrium is stressed the system works to restore equilibrium.
Le Chatelier's Principle: Changing Concentration
CO(2) dissolves in our blood to produce carbonic acid.
CO(2) + H(2)O <---> H(2)CO(3)
During exercise the concentration of CO(2) in our blood increases. This increases the rate of the forward reaction.
To keep the acid level within a safe range we breathe more rapidly. This reduced the CO(2) level and shifts the reaction back to the left.
Le Chatelier's Principle: Changing Temperature
Heat may be considered as a reactant or product
In an exothermic reaction, if the system is heated the reaction is pushed to the left
2SO(2) + O(2) <---> 2SO(3) + Heat
In an endothermic reaction , if the system is heated the reaction is shifted to the right
H(2) + I(2) + Heat <---> 2HI
- When temperature is increased endothermic reaction is favoured
- When temperature is decreased exothermic reaction is favoured
Le Chatelier's Principle: Changing Pressure
The equation coefficients tell you the numbe rof gas particles on each side.
- Increasing the pressure favours the side with least particles
- Decreasing the pressure favours the side with most particles
Example:
N(2) + 3H(2) <---> 2NH(3)
4 moles 2 moles
Increasing the pressure shifts teh reaction to the right. Decreasing the pressure shifts the reaction to the left.
Questions: Part 1
A deep undergound cavern contains 2,24 x 10^6L of methane gas (CH(4)) at a pressure of 1.50x 10^3 kPa and a temperature of 315K.
a) How many Moles of CH(4) does the cavern contain? (Answer = 1 283 594mol)
b) How many kilograms of CH(4) deas the cavern contain? (Answer = 20 538kg)
48g of oxygen gas (O(2)) occupies a volue of 21L at a temperature of 20*C. What pressure is the gas exerting? (in kPa) (Answer = 173.9 kPa)
Determine the total pressure of 3 gases mixed together in a container. The partial pressures are: P(O(2))=30.0kPa P(N(2))=56.7kPa P(H(2))=25.3kPa (Answer = 112kPa)
Questions: Part 1
A deep undergound cavern contains 2,24 x 10^6L of methane gas (CH(4)) at a pressure of 1.50x 10^3 kPa and a temperature of 315K.
a) How many Moles of CH(4) does the cavern contain? (Answer = 1 283 594mol)
b) How many kilograms of CH(4) deas the cavern contain? (Answer = 20 538kg)
48g of oxygen gas (O(2)) occupies a volue of 21L at a temperature of 20*C. What pressure is the gas exerting? (in kPa) (Answer = 173.9 kPa)
Determine the total pressure of 3 gases mixed together in a container. The partial pressures are: P(O(2))=30.0kPa P(N(2))=56.7kPa P(H(2))=25.3kPa (Answer = 112kPa)
Showing Pressure Changes Graphically (TO BE FINISH
Showing Temperature Changes Graphically (TO BE FIN
Showing Concentration Changes Graphically (TO BE F
Dynamic Equilibrium
- Rate of foward reaction = rate reverse reaction
- No observable change
- Both direction of reaction are occuring
Example: What effect do each of the following cahnges have on equilibrium position for this reversible reaction.
PCl(3) + heat <----> PCl(3) + Cl(2)
a) Addition of Cl(2) - a product shifts the equilibrium to the left to reduce the amount of Cl(2)
b) Increase in pressure - The equation has 1 gas reactant particles and 2 has product particlles. The increase in pressure is relieves if the equilibrium is shifted to the left
c) Removal of heat - as heat is a reactant, the removal of heat will shift the reaction to the left. This direction is heat producing.
d) Removal of PCl(3) as it is formed - The removal of the product PCl(3) shifts the reaction tot he right to make mroe PCl (3)



Comments
No comments have yet been made