BONDING - GCSE
- Created by: WillyG12356
- Created on: 01-02-24 18:03
Fullscreen
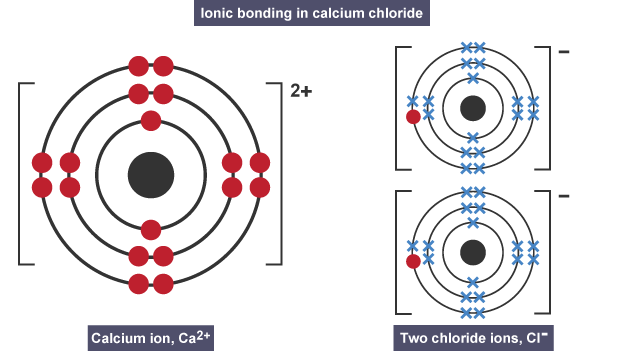
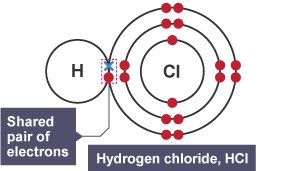
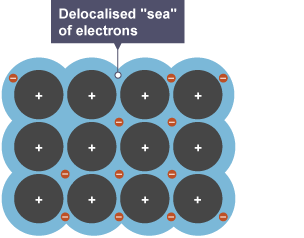
IONIC BONDING
COVALENT BONDING
METALLIC BONDING
DEFINTION
The transfer of electrons between a metal and a non-metal to form strong electrostatic forces.
The shared pair of electrons forming a strong covalent bond.
The strong electrostatic attraction between cations and delocalised electrons.
DIAGRAM
M/B POINT
All ionic compounds have high melting and boiling points due to the strong electrostatic forces between ions. This means that it takes a lot of heat energy to break them apart.
Simple molecular substances have very low melting and boiling points and are gases at room temperature. This is because the intermolecular forces are…
Comments
No comments have yet been made