1.2: Stable & Unstable Matter
- Created by: TDizzle27
- Created on: 10-09-19 22:22
What are the types of radiation?
Alpha - A helium nucleus (2 protons & 2 neutrons)
Beta - high-speed electron/positron
Gamma - an electromagnetic wave
Atoms & Radiation
Some isotopes of atoms are stable. This means that they do not need to change and don't give off radiation, e.g. carbon 12
Heavier elements are more unstable than lighter ones. Carbon 14 is radioactive as it has too many neutrons, causing it to decay.
Forces
Protons are all positively charged so they attempt to repel each other by electrostatic repulsion. They are kept together by the strong nuclear force.
Four fundamental forces:
- Strong nuclear force
- Weak nuclear force
- Gravitational force
- Electromagnetic force
Strong nuclear forces act between nucleons (P-N, P-P, N-N). It has a range of 3 femtometers (3 x 10^-15 m).
The strong nuclear force is attractive down to around 0.5fm, where it becomes repulsive (this prevents protons/neutrons being pushed into each other).
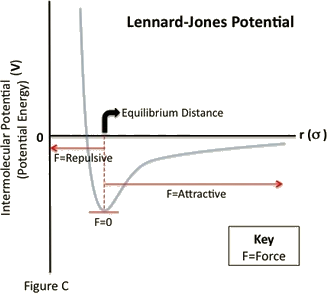
This graph shows the relationship between distance (x-axis) and the attractive/repulsive force (y-axis).
Some isotopes of atoms are unstable & give off radiation all the time. They are radioactive. We cannot alter this…
Comments
No comments have yet been made