Topic 1 - Atomic Structure and the periodic table
All of the key concepts of topic 1 AQA GCSE chemistry
- Created by: Ana Rojas
- Created on: 21-10-17 19:03
Elements and compounds
An Element is a substance that cannot be broken down chemically.
A compound is a substance that contains at least two different elements, chemically combined in fixed proportions. when chlorine reacts to make a compound it chemically combines and becomes a chloride.
-ide and -ate Rules:
Rule 1 – if the compound name ends in –ide, then it usually contains only two elements.
For example: Calcium + Oxygen → Calcium oxide.
Rule 2 – if the compound name ends in –ate or –ite then it contains three or more elements one of which is always oxygen.
For example: Calcium + Carbon + Oxygen → Calcium carbonate.
Rule 3 – The element to the furthest left in the Periodic Table comes first.
Atoms,formulae and equations
- Elements are mad up of atoms that are all the same.
- compounds are made from atoms (or charged atoms) of different elements which have been chemically joined together.
- CH3COOH is a compound commonly known as vinegar , its chemical name is ethanoic acid, is made of three elements, these are carbon,hydrogen and oxygen, these atoms are joined by sharing electrons. if two or more atoms are joined together by sharing their electrons the atoms form a molecule.
- you can see which elements make up a compund by looking at its formula,for example , the compound magnesium oxide, MgO contains Mg(magnesium) and O2 (oxygen).
Mixtures
- a mixture consists of 2 or more elements or compounds NOT chemically combined together. The chemical properties of eac substance in the mixture are unchanged. mixtures can be separated by physical processes. these processes do not involve chemical reactions.
- These separation processes include:
- filtiration
- crystallisation
- distillation
- chromatography
- Fractional distillation works by using a tall tower of gaps and surfaces, which gradually get colder towards the top. the liquid mixture is heated at the bottom and the liquids boil together to amke a mixture of gases. as each gas reaches a surface at the same temperature as its boiling point (or condensing point) that gas will condense and the condensed liquid will run off. the other gases continue up through the gaps until they reach nthe surface at their condensing temperature. eventually nearly all the gases in the mixture will condens and be collected as separated liquids. the final gas is left at the top of the tower and is collected as a gas.
The Structure of an Atom
- Individual atoms are very small. there are about ten million on this full stop.
- an atom is made up of a nucleus that is surrounded by electrons.
- the nucleus carries the positive charge.
- electrons, which surround the nucleus, each carry a negative charge.
- electrons occupy the space around the atom the nucleus in 'shells'. the space between the nucleus and the electron shell is empty space.
- the nucleus contains most of the mass of an atom, and the electrons contribute very little.
- the nucleus of an atom is made up of protons and neutrons., protons have a positive charge and neutrons have no charge.
- the atomic number is the number of protons in an atom. the atomic number for helium is 2 because it has 2 protons.
- the nucleus is made up of particles that are much heavier than electrons. the relative masses and charges of electrons,protons and neurons are shown below:
- ELECTRONS have a relative charge of -1 and a relative mass of 1/2000
- PROTONS have a realtive charge of +1 and a relative mass of 1
- NEUTRONS don't have a realtive charge and have a relative mass of 1
- if an atom has an atomic no. of 3 and a neutral charge then it ust be a Li atom. it is neutral because it has 3 P's and 3 E's. if the Li atom loses one negativley charged electron, then it becomes a charged particle (ion) with a charge of +1 as there is one more P than E
Sub-atomic particles and isotopes
- the atomic number is the number of protons in an atom
- the mass number is the number of protons and neutrons in an atom.
- if the atomic number of a particle is 11 and the mass number is 23 and a neutral charge, it must have 11 P's,11 E's and 12 N's (23-11)
isoptopes:
- the relative atomic mass of an element is the average mass of the different isotopes of an element. Chlorines Ar =35.5 because of this. we use the relative abundance calculation:
- Ar= (mass of 1st isotope x % of 1st isotope)+(mass of 2nd isotope x % of 2nd isotope) all divided by 100
Electronic structure
- Electrons occupy shells around the nucleus. the 1st shell can hold up to 2 electrons and the 2nd shell onwards can hold up to 8 electrons. but for the first 20 elements we should learn go up to the 3rd shell. E.G. oxygen ahs an stomic no. of 8, meaning the first 2 electrons go into the 1st shell and the other 6 electrons go into the 2nd shell. it can presented as: 2,6.
- There is a link between the arrangement of elements in the periodic table and electronic structure. E.G. let us consider an atom of and element that has the electronic structure of 2,8,6.
- This element has 3 electronic shells , so it is in the third row of the periodic table.
- it has six electrons in its outer shell, so it is in group 6 (the 6th column)
- using the periodic table, we can find that its atomic no. is 16 and its element is sulphur, S.
- we can also use a dot and cross diagram to represent the electronic structure of an element.
- you have to fill a shell before going into the next.
Comparing non-metals and metals
- most metals are instantly recognisable as they are shiny whereas non-metals aren't.these are the other physical properties of metals and non-metals:
- METALS - lustrous,hard(with the exception of mercury),high density, high tensile strength, high melting and boiling point, good conductors of heat and good electrical conductivity.
- NON-METALS - dull,soft/brittle/liquids or gases,low density,low or no tensile strength or gas,low melting and boiling points,poor or no thermal conductivity, poor or no conductors of electricity(with the exception of carbon)
- chemical properties of metals are the result of reactions qith oxygen or acids. although copper is resistant to attack by oxygen and acid, (which is the reason it is good for saucepans, many other metals react ith oxygento make an oxide such as calcium oxde and iron oxide. metals react with acids to make salts and hydrogen gases.metals form basic oxides
- one chamical property of non-metals is the the result of the reaction with oxygen. Carbon reacts with oxygento make carbno dioxide. Carbon dioxide dissolves in water to make midly acidic solution. this is what fizzy water contains. non metals form acidic (or neutral) oxides.
Comparing metals and non-metals part 2
- When a metal, such as magnesium, reacts with a non-metal such as oxygen, the metal loses electrons and the non- metal gains electrons. this is because metal atoms have few outer electrons which they 'lose' to form a positive ions. oxygen accepts the electrons.
- When a non-metal such as chlorine, reacts with ametal such as sodium, the non-metal gains an electron from the metal. this is because non-metal atoms have empty spaces in their outer shell in which they ' gain' other electrons from metals to form negative ions. The non-metal does not form positive ion.
- in summary metals form positive ions and non-metals for negative ions.
KEY CONCEPT - The outer electrons
Stable Atoms - The noble gases are all very unreactive. Their atoms are very stable and don't normally react with other atoms. This is because their outer shells contain eight electrons ( except He, which contains two electrons). This stable number of electrons in the outer shell means there is no tendency to transfer electrons.
Less stable atoms - All other atoms are less stable. Their electrons move or share with other electrons to try to become stabe as the atoms with 8 electrons in their outer shell, chemical reactivity and chemical reaction depend on the no. of electrons in the outer shell,(although not always true in the transition metals). three things can happen to electrons:
- they can be transferred to other atoms.
- they can be gained.
- they can be shared between another atoms
Transferring or sharing ?
if an atom has one or two electrons in its outer shell these electrons will transfer out.if an atom has 6 or 7 electrons in its outer shell, electrons from other atoms will add in to the 'spaces' to make a stable outer shell. they will transfer in from the outer shell of another atom.
if an atom hs an unstable no. of electrons i.e. 3,4 or 5, in its last shell, this atom will share its electrons with electrons from other atoms. common e.gs are carbon, hydrogen and oxygen
Exploring group 0
Patterns in group 0 : all the elements of group 0 in the periodic taable have 2 things in commmon, they are all unreactive and they are all gases. the boiling points of the elements in group 0 show a trend; Helium has the lowest boiling point, this means the atoms of the element kepp moving raidly (as a gas) at lower temperatures than the atoms of xenon. the trend is that the boiling points of the gases increase down the group.Therefore, boiling points increase as atomic mass increases.
Why does He stay as a gas at lower temperatures? all the elements in group 0 are gases. These gases exist as single atoms,not molecules. The smaller the atom the 'easier' it is for it to keep moving around rapidly.So at lower temperatures the small atoms of helium move more easily than the larger atoms of krypton. So krypton has a higher boiling point than helium.
Why do elements in group 0 exist as single atoms? the elements of group 0 don't generally make compounds with other elements and are unreactive. They don't make compounds because the atoms have 8 electrons in their outer shell.This is a very stable configuration. So there is no electron movement from one atom to another.
Exploring group 1
Properties of group 1 elements : lithium, sodium and potassium are group 1 elements that are less dense than water. all three metals would floaton the surface when added to water. G1 metals react vigorously with water and make hydrogen. G1 metals also burn in oxygen to form oxides. Sodium burns to make sodium oxide.
Alkali metal + water = Alkali metal hydroxide + hydrogen
Making ions: Alkali metals have similar chemical properties. this is because when they they react, their atoms need to lose one electron to form the electronic structure of a noble gas. this is known as a stable electronis structure.
when the atom loses one electron it form an ion. it has one more positive charge in its nucleus than negative charges surrounding it.so it is now a positive ion that carries a charge of 1+
Sodium reacts with chloring to make sodium chloride. the sodium makes and ion that carries a 1+ charge. it makes an ionic compound. the compound is a white solid that dissolves in water to form a colourless solution.the other alkali metals make these compound too.
Exploring group 7
The Halogens: Group 7 elements are called halogens. the halogens all have 7 electrons in their outer shell and so all share similar chemical properties. they are non-metals and exist as pairs of atoms eg. Cl2 and F2. They react vigorously with metals such as sodium,potassium and magnesium to produce salts. Halogens react with non-metals to make gases or liquids such as acids.
Group 7 trends: there is a trend in the physical appearance of the halogens at room temperature. Chlorine is a gas and iodine is a solid. as you go down group 7 there is decreasing reactivity.
Displacement reactions of halogens: the reactivity of the halogens decreases down the group therefore if halogens are bubbled thtrough solutions of metal halides there are two possibilities; no reaction or a displacement reaction. if chlorine is bubbled through potassium bromide solution a displacement reaction occurs . An orange colour of bromine is seen. this is because chlorine is more reactive than bromine.
Reaction trends and predicting reactions
Opposite trends: The trends in group 1 and 7 are in opposite directions. Reactivity increases down G1 as the outer electron in a potassium atom is further away from the nucleus than in alithium atom so there is 'less pull' on it by the nucleus so it is lost more easily. so potassium is more reactive that lithium
Reactivity decreases down G7 as the electron trying to transfer into a bromine atom is further awat from the nucleus so it transfers in less easily. So bromine is less reactive than flourine.
Trends across the table : the trend across the table is from metallic to non-metallic.the trend acroos and down the table depends on the structure of the atom.In period 2 and 3 across the table the outer electron will increase by one from group 1 with one electron and group2 with 2 electrons.
G1 elemements lose one electron to form a positive ions easily. it is more difficult for elements to lose 2 or 3 electrons so they are less reactive. G7 gain 1 electron to form ions.
Prediciting Reactions: Knowing the position of an element in the periodic table will allow us to predict its behaviour with water,acid or oxygen. EG. Sulphur and phosorus are both non0metals and will likely react with oxygen in a similar way.
Transition Metals
Comparing Properties: all transition elements are metals and have typical metal properties.Transition elements have different reactivities. they are less reactive than elements in group 1.
Catalysts: A transition element and its compounds are often catalysts. Iron is usefd in Haber process to make Ammonia and Nickle is used in the manufacture of margerine to harden oils.
A catalyst is an element or compound that changes the rate od a chemical reaction without taking part in the reaction as a reactant. Catalysts are unchanged by the reaction.
Ions and coloured compounds: a compound of group 1 is usually white. Whereas a compound of a transition element is often coloured EG. copper=blue,iron(II)=pale green), iron(III)= orange/brown. The reason that iron forms compounds with different colours is that it can form two different types of positive ion. Transition metals are able to lose different numbers of electrons.Iron can lose 2 or 3 electrons.
Reactivity Series
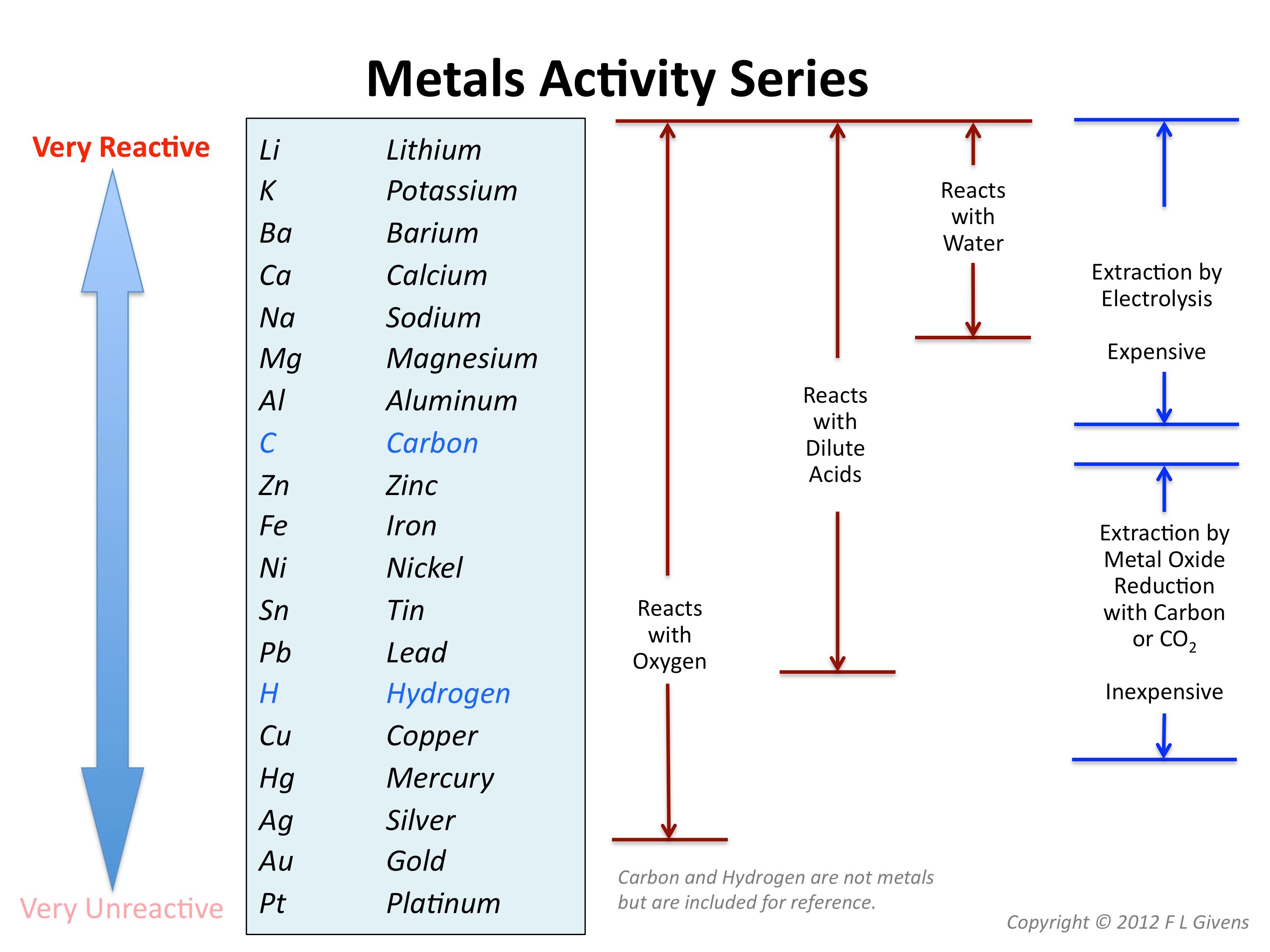
Comments
No comments have yet been made