C2H4(g) + H2O(l) 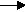 C2H5OH(l)
C2H5OH(l)
exothermic reaction
Temperature...eqm goes left
high yield favoured by a low temperature but it is slow so a compromise of 300°c
Pressure...2 moles to 1 mole
eqm goes right
high yield favoured by high pressure but it is expensive so a compromise of 6.5m Pa
Phosphoric acid, H3PO4 is added to increase the rate of both reactions equally so eqm is unaffected but reaction is much quicker
Comments
No comments have yet been made