Core Organic Chemistry
- Created by: Beth I
- Created on: 03-05-20 20:05
Definitions
General Formula: Algebraic formula to describe any compound in the functional group e.g. CnH2n+1OH = alcohols
Spec Definition: the simplest algebraic formula of a member of a homologous series
Molecular Formula: Actual number of atoms in a compound e.g. C4H9OH
Structural Formula: Shows the arrangement of atoms carbon by carbon. E.g. CH3CH2CH2CH2OH
Spec Definition: the minimal detail that shows the arrangement of atoms in a molecule
Skeletal Formula: Shows the bonds of the carbon skeleton only, with any functional groups.
Spec Definition: the simplified organic formula, shown by removing hydrogen atoms from alkyl chains, leaving just a carbon skeleton and associated functional groups
Definitions
Displayed Formula: Shows how all of the atoms are arranged and all of the bonds between them.
Spec Definition: the relative positioning of atoms and the bonds between them
Homologous Series: a series of organic compounds having the same functional group but with each successive member differing by CH2
Functional Group: a group of atoms in a molecule responsible for the characteristic reactions of a compound
Alkyl Group: a side chain on an organic compound, of formula CnH2n+1 -can bond to the main carbon chain
Saturated: single carbon-carbon bonds only
Unsaturated: the presence of multiple carbon-carbon bonds, including C=C and aromatic rings
Types of Carbon Skeleton
There are different types of Carbon Skeleton:
Aliphatic: a compound containing carbon and hydrogen joined together in straight chains, branched chains or non-aromatic rings
Alicyclic: an aliphatic compound arranged in non-aromatic rings with or without side chains
Aromatic: a compound containing a benzene ring
Nomenclature
Compounds have systematic names based on certain rules.
The stem comes from the number of carbons in the longest continuous carbon chain
The functional group will tell you what homologous series it is- giving a prefix or suffix
Number the longest carbon chain so that the functional group has the lowest number possible e.g. If the group is on the end, it is number 1
If there is more than one long chain, use the one with the most side chains
Any side-chains or less important functional groups are added as prefixes at the start of the name.
o Put them in alphabetical order, after the number of the carbon atom each is attached to
o If there is more than one identical side chain or functional group, use di (2), tri (3), or tetra (4) before that part of the name but ignore this when working out the alphabetical order.
Isomers
Isomers are compounds with the same molecular formula but a different arrangement of atoms
Structural Isomers: compounds with the same molecular formula but different structural formulae
There are 3 types of structural isomers;
Chain isomers: Where the carbon skeleton can be arranged differently, i.e. As a straight chain or branched in different ways
These isomers have similar chemical properties- but their physical properties, like boiling points, will differ due to the change in shape
Positional Isomers: where the skeleton and the functional group could be the same, only with the functional group attached to a different Carbon- They have different physical properties and the chemical properties may also differ
Functional Group Isomers: where the same atoms can be arranged into very different functional groups- They have very different physical and chemical properties
Fission
Bond breaking is called fission and a covalent bond can be broken in one of 2 ways:
Homolytic Fission:
This is when the electrons in the covalent bond go back to their original atom.
The movement of 1 electron is shown by a half-headed arrow because electrons are normally shown moving in pairs- shown by a full arrow.
Heterolytic Fission:
This is when both of the electrons in the bond go to one of the atoms
This results in 2 ions- one positive and one negative. The negative ion is a Free Radical.
Reactants
Reactants then start a reaction going.
There are 3 types of reactants:
Free Radicals are atoms or groups of atoms with an unpaired electron. They are extremely reactive and are said to be 'short-lived'.
Electrophiles are usually negative ions but they must have a lone pair of electrons which are donated to form a new covalent bond
Nucleophiles are electron-pair donors
Types of Reactions
Addition Reactions: involves 2 molecules joining to become 1 molecule
Substitution reactions: involves an atom, or group of atoms, being replaced by another atom (or group)
-> 2 molecules make 2 new molecules
Elimination Reactions: Involves the removal of one molecule from another
-> 1 molecule gives 2 molecules.
Alkanes
Alkanes are saturated hydrocarbons with the general formula: CnH2n+2.
They have only single covalent bonds. These types of covalent bonds are called sigma bonds (σ-bond)
A sigma bond is the result of the overlap of 2 orbitals, one from each bonding atom. Each overlapping orbital contains a single electron, so the sigma bond has 2 electrons.
Each carbon atom has 4 sigma bonds, either C - C or C - H. Repulsion between the electron pairs results in a 3D tetrahedral arrangement around each Carbon. This allows the atoms to rotate freely around the bond
Properties of Alkanes
Between the molecules of Alkanes, there are induced dipole-dipole interactions. The longer the carbon chain, the stronger the London forces because there's more surface contact and more electrons to interact. So, as the molecules get longer, more energy is needed to overcome the London forces, so the boiling point increases.
A branched alkane has a lower boiling point than it's straight chain isomer because they can't pack closely together and they have smaller molecular surface areas- so the London forces are weaker.
The C - C or C - H bonds are relatively strong and non-polar. Alkanes are therefore unreactive towards acids, alkalis, electrophiles and nucleophiles but they do combust in air or oxygen- making them important fuels
Combustion
Combustion reactions happen between gases, so liquid alkanes have to be vapourised first. Smaller gases are more volatile, so they'll combust more easily.
Larger alkanes release more energy though- due to having more bonds.
Complete Combustion:
Hydrocarbon + Oxygen -> Carbon Dioxide + Water
Incomplete Combustion:
Occurs when there is an insufficient amount of oxygen available.
Hydrocarbon + Oxygen -> Carbon Monoxide + Water
Carbon monoxide is dangerous and carbon monoxide poisoning can be fatal.
Alkane Reactions with Halogens
Alkane + Halogen ->Halogenoalkane + Hydrogen Halide (g)
UV Radiation
- Explosive reaction
- Substitution reaction
1 hydrogen is substituted for a halogen and a hydrogen halide gas is given off.
Free Radical Substitution Mechanism
Occurs between an alkane and a halogen
Initiation:
Homolytic fission of the Cl - Cl bond.
Propagation:
Free radicals take a hydrogen to form a hydrogen halide, leaving an alkyl free radical.
The alkyl free radical then combines with a halogen atom forming a haloalkane.
The free radical produced in each reaction triggers another reaction and so on- a chain reaction.
Termination:
When two free radicals combine, they create a stable molecule- ending the reaction.
There are a multitude of termination reactions
Free Radical Substitution Mechanism
Issues with Free Radical Substitution
The problem with Free Radical Substitution is that you get a mixture of products, so it isn't an effective way to get a particular product.
-> if there is an excess of halogen atoms, when the haloalkane is produced at the termination stage, it can then react with a halogen radical and form a different product. It would therefore be necessary to instead have an excess of the alkane to reduce this risk.
Free radical substitution can also take place at any point along the carbon chain, so any number of isomers can be formed.
Inefficient and hard to control -> not good for industrial use.
Alkenes
Alkenes are unsaturated hydrocarbons with the General formula: CnH2n.
They have single covalent bonds and double covalent bonds.
Double Bonds
A double bond is made up of a sigma bond and a pi (π) bond.
The sigma bond is formed when 2 orbitals overlap in a straight line, giving the highest possible electron density between the nuclei. This means there is a strong electrostatic attraction between the nuclei and the shared electrons- this is a single covalent bond. They have a high bond enthalpy, making them the strongest type of covalent bond.
A pi bond is formed by the sideways overlap of 2 adjacent p-orbitals. Because p-orbitals are dumb-bell shaped when they overlap they form 2 parts, one 'above' the other 'below' the molecular axis, spreading out the electron density- they have a weaker electrostatic attraction and a relatively low bond enthalpy.
Stereoisomerism
Stereoisomers have the same structural formulas but a different arrangement in space.
A type of stereoisomer is E/Z isomerism.
They occur due to the lack of rotation around the double bond. When two double bonded carbon atoms each have two different atoms or groups attached to them, one is called the 'E-isomer' the other the 'Z-isomer'.
The 'Z isomer' has the same groups either both above or both below the double bond, whilst the 'E-isomer' has the same groups positioned across the bond.
To remember which is which is that in Z-isomers the groups are on 'ze zame zide' whereas in E-isomers the groups are 'enemies' on opposite sides.
CIP Rules
You can work out which isomer the alkene is even if the groups aren't the same:
Look at the atoms directly bonded to each of the C=C Carbon atoms. The atom with the higher atomic number on each carbon is given the higher priority.
Number the higher priority atom 1 and the lower priority atom 2 on both Carbon atoms.
Now you can assign the isomers as E- and Z- as before, by looking at how the groups of the same priority are arranged.
If both carbons have priority number 1 or priority number 2 on the same side, then it is a Z-isomer. If not then it is an E-isomer.
If the atoms in the groups are the same then you have to look at the next atom in the groups to work out which has the higher priority.
Cis-Trans Isomers
E/Z isomers can also be called Cis-Trans Isomers.
'Cis-isomers' are where the same groups are on the same side of the double bond (Z-isomers)
'Trans-isomers' are where the groups are on the opposite sides of the double bond (E-isomers)
If the carbons have totally different groups attached the cis/trans naming system doesn't work as there is no way of deciding which one is cos and which one is trans. The E/Z system still works though.
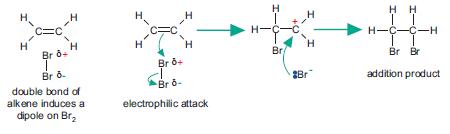
Electrophilic Addition Mechanism
-> involves the reaction of any alkene and a molecule such as H2 or a Halide.
Molecules such as Br2 are non-polar. When they are near a double bond a dipole-dipole (London) force is induced- electrons in the Br2 are repelled away from the bond.
-> attraction between the δ+ of the Br and the π bond of the = bond.
-> the π bond then joins with the δ+ Br
-> Electron pair between the Br atoms is repelled completely to the δ- Br and the bond between them breaks
-> the electron-deficient Br- the δ+ Br- is the electrophile as it then accepts the π electron pair.
THIS IS HETEROLYTIC FISSION
-> BOTH OF THE ELECTRONS GO TO ONE OF THE REACTANT ATOMS, THE OTHER GETS NOTHING
As a result of this an intermediate is formed (a carbocation)
Electrophilic Addition Mechanism
Hydrogenation
Alkene + H2 -> Alkane
TEMP 150°C
Ni Catalyst
-> has industrial uses e.g. In the food industry
-> carboxylic acids with double bonds into carboxylic acids without double bonds
ISSUES: can create trans-fats which raise LDL levels and can cause heart disease
Alkenes with Halogens
Alkene + Halogen -> Haloalkane
no specific conditions
If with Bromine- goes from orange to colourless
-> used as a way to test for alkenes/ C=C
Alkenes with Hydrogen Halides
Alkene + Hydrogen Halide -> Haloalkane
No specific conditions
Hydration (Alkenes with Steam)
Alkene + H2O -> Alcohol
TEMP 100°C
PHOSPHORIC ACID/
SULPHURIC ACID CATALYST
Haloalkanes
A haloalkane is a saturated molecule with the general formula: CnH2n+1X where X is a halogen.
Halogens are generally much more electronegative than Carbon, making the Carbon - Halogen bond polar.
The δ+ carbon is electron deficient, meaning it can be attacked by a nucleophile.
A nucleophile is an electron pair donor- it could be a negative ion or an atom with a lone pair of electrons. OH-, CN- and NH3 are all nucleophiles which react with haloalkanes- forms a dative covalent bond.
Hydrolysis of Haloalkanes (Nucleophilic Substituti
Haloalkanes can by hydrolysed into alcohols- this is a nucleophilic substitution reaction
It can either be done with aqueous hydroxides or by water in the presence of AgNO3 and ethanol.
Change in functional group: haloalkane alcohol
Reagent: potassium (or sodium) hydroxide
Conditions: In aqueous solution; Heat under reflux
Mechanism: Nucleophilic Substitution
Type of reagent: Nucleophile
OH- is the nucleophile which provides a pair of electrons for the Cδ+. The C - Br bond breaks heterolytically- both electrons are taken by the Br-.
Nucleophilic Substitution
Water can also act as a nucleophile but as it is only a weak nucleophile the reaction is a lot slower than the one above.
The general equation is:
R - X + H2O -> R - OH + H+ + X-
The rate of hydrolysis of haloalkanes depends on the Bond Enthalpy. Weaker Carbon - Halogen bonds (those with lower bond enthalpies) break more easily so they react faster.
Iodoalkanes have the weakest bonds so they hydrolyse the fastest, whereas Fluoroalkanes have the strongest bonds so they take longer to hydrolyse.
Environmental Concerns due to use of haloalkanes
Chlorofluorocarbons were used a lot as they are stable, volatile, non-flammable and non-toxic.
They were banned once it was discovered that they were destroying the ozone layer.
Ozone in the upper atmosphere absorbs a lot of the UV radiation from the sun, it's formed naturally when an oxygen molecule is broken down into 2 free radicals by UV radiation. The free radicals then attack other oxygen molecules to form ozone.
In the 70s and 80s it was discovered that the ozone layer was getting thinner rapidly over Antarctica and the Artic, allowing more UV radiation to reach Earth. The holes were formed because CFCs in the upper atmosphere absorb UV radiation and split to form chlorine free radicals. These free radicals catalyse the destruction of the ozone- they destroy ozone molecules and are then regenerated to destroy more molecules.
The chlorine free radical is regenerated and it goes straight on to attack another ozone molecule. One chlorine atom can destroy 10,000 ozone molecule before becoming a stable molecule.
So the overall reaction is: O3 (g) + O (g) -> 2O2 (g) with Cl• as the catalyst
Alternatives to CFCs
- HCFCs (hydrochlorofluorocarbons) and HFCs (Hydrofluorocarbons) are used as temporary alternatives
- Hydrocarbons are also used
- HCFCs break down in the atmosphere within 10-20 years and whilst they still damage the ozone layer, they have a much smaller effect.
- HFCs also break down in the atmosphere but as they don't contain Cl they don't affect the ozone layer.
Unfortunately, these alternatives are greenhouse gases and are about 1000x worse than carbon dioxide.
Nowadays, most aerosols have been replaced by pump spray systems or use nitrogen as the propellant.
Related discussions on The Student Room
- A-level chemistry. Draw a labelled diagram to show the formation of the π-bond. »
- uni modules and how they apply to my course »
- Is my degree unusually difficult or am I bust? »
- Required practicals chemistry A level »
- I enjoy chem practicals more than bio practicals. Is a chem degree right for me? »
- Leeds vs Sheffield vs York chemistry »
- Undergraduate chemistry »
- Uni of Manchester pharmacy help »
- Forced to drop an a level »
- Grade Growth Chronicles | From C's to A's (23-24) »


Comments
No comments have yet been made