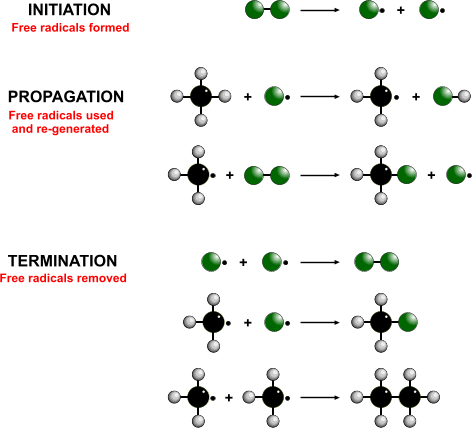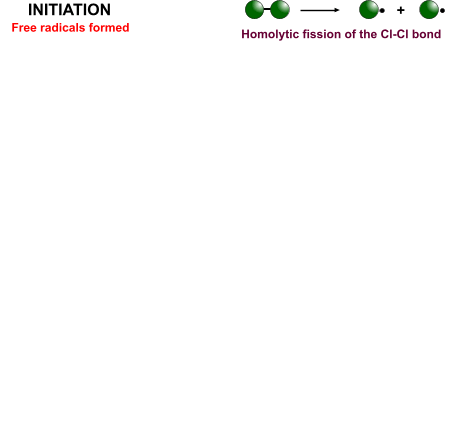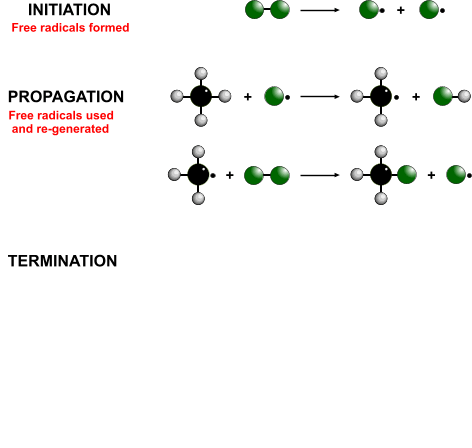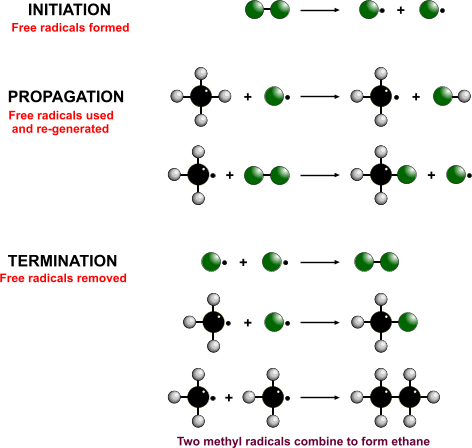
When a mixture of a halogen and alkane are exposed to sunlight, a haloalkane forms
Free-radical substitution
- Initiation - Breaking of the diatomic halogen molecule into two free radicals
- Propagation - The free-radical takes a hydrogen from the alkane to form a hydrogen halide and another free radical
- Termination - Two free radicals join to form a stable molecule
Comments
No comments have yet been made