transition metals and their react with aqeous ions
- Created by: elisha chopra
- Created on: 07-11-16 11:10
Fullscreen
Optical isomers
Optical isomers can form optical isomers. Optical isomers occur when:
- 3 molecules or ions of a bidentate ligand form an octahedral complex
- 2 molecules or ions of a bidentate or a monodentate molecules or ions bind to the transition metal
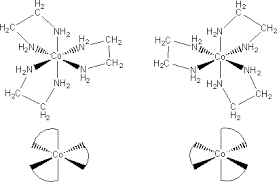
Optical isomers have the same colour and chemical property but differ in the way that they rotate the plane of polarization of polarised light
- cis= same (on the same fae of thew octahedral and can also be called Z-isomers)
- trans= different (on oppasite or different faces of the octahedral and can also be called E-isomers)
Aqeous Fe(II) and Fe(III) ions
aqeous fe(III) ions are:
- more acidic than Fe(II) ions
- act as a Bronstead-Lowery acid (proton donar)
- have high charge denisty
- high charge density of metal ion attracts electron denisty from oxygen atom in H2O ligand
The high charge density in the Fe(III) ions causes and iductive effect in the structural bonding and…
Comments
No comments have yet been made