Topic 1A
5.0 / 5 based on 1 rating
- Created by: DBaruch
- Created on: 12-04-16 18:33
Monomers and Polymers
- The majority of carbohydrates, proteins and nucleic acids are polymers.
- Polymers are large, complex molecules that are made of large chains of monomers that are joined together
- Monomers are small, basic molecular units that can form into a polymer
- Examples of monomers include monosaccharides, amino acids and nucleotides
1 of 23
Making and breaking polymers
- Most polymers that are biological are formerd from there monomers from condensation reactions
- A condensation reaction creates a chemical bond between monomers and in the process of this releases a water molecule
- Biological polymers are broken down into their monomers by a hydrolysis reaction
- This breaks the bond between the monomers using a water molecule .
2 of 23
Monosaccharides
- Every single carbohydrate contains carbon, hydrogen and oxygen.
- The monomers that carbohydrates are made from are monosaccharides
- Glucose is a hexose sugar meaning that it has 6 carbon atoms.
- There are 2 types of glucose, alpha and beta
- They are isomers (the same formula but a different structure)
3 of 23
Disaccharide formation
- A disaccharide forms when 2 monosaccharides join together.
- This occurs using a condensation reaction
- A glycosidic bond forms because water is released
- Sucrose is made from a glucose and a fructose.
- Lactose is made from a glucose and a galactose
- Maltose is made from 2 glucose molecules
4 of 23
Benedicts test for sugars
- Reducing sugars include all monosaccharides and some dissacharides (maltose and lactose)
- You add benedicts reagent (CuSO4) to a sample and heat it using a water bath
- A postive test will see the sample go from blue-green-yellow-orange-brick red
- The higher the concentration of reducing sugar the further the colour change goes.
- A more accurate way to compare the amount of reducing sugar in each sample is to remove the colour precipitate and use a colorimiter to test to absorbance of the remaing benedicts reagent
- Even if there is not a colour change then a non reducing sugar may still be present
- To test for non reducing sugars you need to break the sample down into the individual monosaccharides.
- To the new solution add HCl and heat in a water bath
- After heating the sample needs to be neutralised by adding sodium hydrogencarbonate.
- Then heat the sample again adding the benedicts solution and carrying out test as you would for reducing sugars.
- The colour given will either be positive (green to red) or negative (stays blue)
5 of 23
Polysaccharides
- A polysaccharide is formed when more than 2 monosaccharides are joined together by condensation reactions and creates a glycosidic bond
- Polysaccharides can be broken back into their monosaccarides by a hydrolysis reaction
6 of 23
Functions of polysaccharides- Starch
- Cells get energy from glucose
- Plants store there excess energy as starch
- Starch is a mixture of amylose and amylopectin which are polysaccharides from 2 alpha glucose molecules
- Amylose is long, unbranched chain of alpha glucose, the angles of the glycosidic bonds give it a coiled structure, this means that it is compact and makes it good for storage as you can fit lots into a small space
- Amylopectin is long, branched chain of alpha glucose, it has side branches which allow the enzymes that break it down to reach the glycosidic bonds easily, this allows the glucose to be released easily
- Starch is insoluble in water and doesn't affect the water potential, this means that water cannot enter the cell which would make the the starch swell.
7 of 23
Functions of polysaccharides- Glycogen
- Animals store their excess glucose as glycogen
- It is long and branched with lots of side branches coming off of it
- The side branches allow energy to be released quickly
- It is very compact meaning that, alot can be stored in a small space.
8 of 23
Functions of polysaccharides- Cellulose
- Cellulose is long, unbranched chains of beta-glucose.
- When the glucose molecules join they form straight cellulose chains.
- The chains are linked by hydrogen bonds and form strong fibres called microfibrils
- The strong fibres provide structural support for cells (in plant cell walls)
9 of 23
Iodine test for starch
- To test for starch add iodine dissolved in potassium iodide solution to a sample
- If there is starch the sample will change from browny-orange to a blue-black
10 of 23
Lipids and testing for lipids
- Lipids all contain hydrocarbons and the other componets relate to the lipids function
- To test for lipids you carry out an emulsion test
- Shake the substance in a test tube with ethanol for 1 minute
- In the presense of a lipid a the solution will turn a milky colour
- The stronger the lipid, the more prominent to the milky colour will be
11 of 23
Triglycerides
- Triglycerides have molecule of glycerol(C3H8O3) and 3 fatty acids "tails"
- The tails are hydrophobic meaning that they repel water molecules also this makes triglycerides insoluble in water.
- All fatty acids have the same basic strucutre but the hydrocarbon tail varies
- Saturated fatty acids dont have any double bonds between the carbons
- Unsaturated fatty acids have double bonds between the carbons which cause the chain to kink.
12 of 23
Triglyceride formation and Phospholipids
- Triglycerides are formed by condensation reaction
- An ester bond forms between a fatty acid and a glycerol, this releases a molecule of water
- This happens 2 more times to form a triglyceride
- The lipids found in cell membranes are not triglycerides
- Phospholipids are pretty similar to triglycerides except one of the fatty acids is replaced by a phosphate group
- The fatty acid tails are hydrophobic.
13 of 23
Properties of lipids
- Triglycerides are mainly used as energy storeage molecules because the long tails have lots of chemical energy and lots of energy is released when they are broken down
- They are insoluble in water so they do not affect water potential of the cell and cause water to enter the cells by osmosis.
- Triglycerides bundle together and form insoluble droplets in cells because the fatty acid tails are hydrophobic.
- Phospholipids make up the bilayer of cell membranes which control what goes in and out of a cell
- The heads are hydrophillic and their tails are hydrophobic
- This creates a double layer with their heads facing out towards the water.
- The centre of the bilayer is hydrophobic meaning that water soluble substances can't get through easily.
14 of 23
Amino acid structure and What are proteins made of
- Every amino acid has the same general strucutre
- a carboxyl group (COOH), an amino group (NH2) and a carbon-containing R group attached to a carbon atom .
- All living things share 20 amino acids and the difference between them is the carbon-containing R group
- The monomers of proteins are amino acids
- A dipeptide is formed when 2 amino acids join together
- A polypeptide is formed when more than 2 amino acids join together.
15 of 23
Dipeptide and polypeptide formation and testing fo
- Amino acids link together by condensation reactions to create dipeptides and polypeptides
- The bonds formed between each amino acid is called a peptide bond.
- A hydrolysis reaction occurs when dipeptides and polypeptides are broken down into amino acids
- To find out if a substance contains protein you need to carry out the biuret test
- The test solution must be alkaline to do this add a few drops of sodium hydroxide soltution
- Then add copper (2) sulfate solution
- A postive result will turn purple, a negative result will stay blue
16 of 23
Protein structure
- Primary strucutre- is the sequence of amino acids in the polypetide chain
- Secondary strucutre- Hydrogen bonds form between the amino acids in the chain this makes it coil into an alpha helix or beta pleated sheet
- Tertiary structure- The coiled or folded chain is coiled and folded further this creates more bonds between different parts of the chain including hydrogen bonds and ionic bonds, disulfide bridges form when 2 molecules of the amino acid cysteine come close together. For proteins made from a single polypeptide chain the tertiary stucture forms their final 3D structure
- Quaternary strucuture- Some proteins are made from several polypeptide chains held together by bonds. The quaternary structure is a way thses polypeptide chains can be joined together. This forms their final 3D structure.
17 of 23
Protein shape and function
- The shape of a protein determines its function examples of this are;
- Enzymes- They are usually spherical in shape due to the tight folding of the polypeptide chains. They are soluble and are often involved in metabolism.
- Antibodies- They are involved in the immune response and are usually found in the blood. They are made of 2 light polypeptide chains and 2 heavy polypeptide chains bonded together. Antibodies have variable regions and the amino acid sequences vary in these areas.
- Transport proteins- Channel proteins are present in cell membranes. The channels contain hydrophobic and hydrophillic amino acids which cause the protein to fold up and make the channel. The proteins help to move molecules and ions across membranes
- Structural proteins- They are physically strong and consist of long polypeptide chains lying parallel to each other with cross links between them. Examples of them are keratin and collagen.
18 of 23
How enzymes speed up reactions
- A certain amount of energy is needed to start a chemical reaction this is called the activation energy (often provided as heat)
- Enzymes lower the activation energy needed to start a reaction meaning that a reaction could occur at a lower temperature with the use of an enzyme.
- This speeds up rate of reaction
- When an substrate fits into an enzymes active site it forms an enzyme-substrate complex, its this that lowers the activation energy
19 of 23
Models of enzyme action
- The lock and key model shows that only a specific substrate will fit into a certain enzyme as the active site and the substrate have a complementary shape
- The induced fit model helps to explain why enzymes are specific and only bond to one particular substrate.
- The substrate doesn't only have one to be the right shape to fit the active site, it has to change the shape of the active site in the right way
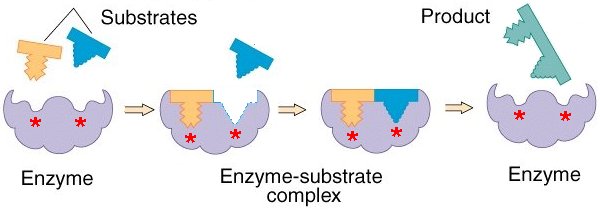
20 of 23
Enzyme properties
- The properties of an enzyme is usually related to their tertiary structure.
- The shape of the active site is determined also by the tertiary structure
- If the substrate shape doesn't match the active site, an enzyme substrate complex won't form and the reaction will not catalyse
- If the tertiary structure is changed in any way, then the shape of the active site will change meaning that the enzyme will no longer be able to carry out its function
- The primary structure of a protein is determined by a gene however mutations can occur and change the tertiary structure of the enzyme formed
21 of 23
Factors that affect enzyme activity
- Temperature- The rate of an enzyme controlled reaction increases when the temperature's increased. More heat means that the molecule will move faster meaning that the substrates are more like to collide with the enzymes active site. If the temperature gets to high the reaction stops this is because enzyme vibrates too much and causes bonds that hold the enzymes shape to change. This means that the active site shape changes and the enzyme and substrate no longer fit together.
- pH- When the pH surrounding the enzyme is either above or below the optimum pH for the specific enzyme then the ionic and hydrogen bonds that hold together the enzymes tertiary structure together meaning the enzyme changes shape and becomes denatured
- Substrate concentration- The higher the substrate concentration the more likely a collision between the enzyme and substrate will occur. However after a certain point (the saturation point) adding more substrate molecules will have no effect on the rate as the enzymes are already busy.
- Enzyme concentration- The more enzymes molecules there are in a soltuion the more likely it is that they will collide with a substrate. However after a point, if the amount of substrate is limited there comes a point in which there are more than enough enzyme molecules to deal with the available substrate.
22 of 23
Enzyme Inhibitors
- Competitive inhibitors have a similar shape to that of a substrate and they compete with substrate molecules to bind to the active site of an enzyme, however when they bind no reaction takes place meaning that no products are formed.
- Non-competitive inhibtors work by binding to the enzyme away from the active site. This causes the active site to change shape meaning that no substrate molecules can bind to it.
23 of 23
Related discussions on The Student Room
- Any good youtube channels for Bio + Chem a levels? »
- Whats beter revising a whole topic for 1 subject or 2 lessons for 2 subjects A level »
- Changing to physical natsci »
- Do I need to know how to draw structures for carbohydrates? (AQA A Level Bio) »
- 25 mark essay question »
- Animal Transport Notes OCR A Level »
- Access to Science course »
- A-level Biology Study Group 2023-2024 »
- For A level Biology students, How reliable/useful are the PMT flashcards? »
- Paper 3 AQA a Level biology »
Similar Biology resources:
0.0 / 5
1.0 / 5 based on 1 rating
5.0 / 5 based on 2 ratings
0.0 / 5
3.0 / 5 based on 3 ratings
3.0 / 5 based on 1 rating
5.0 / 5 based on 1 rating
Teacher recommended
0.0 / 5
3.0 / 5 based on 1 rating
Comments
No comments have yet been made