Nitrogen + Hygrogen 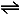 Ammonia
Ammonia
N2 + 3H2 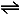 2NH3
2NH3
Haber Process:
- Hydrogen and Nitrogen are mixed together.
- Iron catalyst is used to speed up the reaction.
- Condensed into liquid ammonia to stop the reversible reaction.
- Unused nitrogen and hydrogen are recycled and reused.

Comments
No comments have yet been made