Chemistry minor stuff
- Chemistry
- Organic and Green ChemistryFunctional groups: Alcohols, carboxylic acids and estersFunctional groups: Alkanes, alcohols, carboxylic acids and estersAcids, bases and saltsProperties of hydrocarbonsPaper 2
- GCSE
- AQA
- Created by: Ash
- Created on: 16-05-18 16:56
Alkenes and Hydrogen
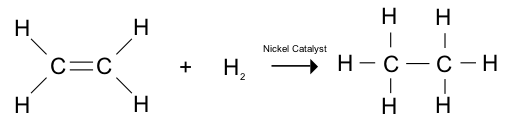
Alkenes and Hydrogen
In the presence of a nickel catalyst, hydrogen can be added to an alkene to give an alkane. E.g. ethene (C2H4) + hydrogen (H2) → ethane (C2H6).
Adding hydrogen atoms across a carbon-carbon double bond is called hydrogenation.
Alkenes and Water
Alkenes and Water
In the presence of a phosphoric acid catalyst, water, in the form of steam, can be added to an alkene to give an alcohol.
E.g. ethene (C2H4) + steam (H2O) → ethanol (C2H5OH).
This can be called a hydration reaction.
Alkenes and Oxygen
Alkenes and Oxygen
Alkenes react with oxygen in combustion reactions. They react in a similar way to other hydrocarbons.
E.g. ethene (C2H4) + oxygen (O2) → carbon monoxide (CO) + carbon (C) + water (H2O)
Because their combustion is incomplete, they burn with smoky flames.
Alkenes and Halogens
Alkenes and Halogens
Alkenes, unlike alkanes, will react when shaken with bromine water. This causes the solution to change colour from orange-brown to colourless.
E.g. ethene (C2H4) (colourless) + bromine (Br2) (orange-brown solution) → dibromoethane (CH2BrCH2Br) (colourless).
This can be used as a test to distinguish between alkenes and alkanes.
Testing for gases
Gas tests
-
Chlorine
-
Insert damp litmus paper into a test tube
-
Litmus paper turns white
-
-
-
Carbon dioxide
-
Bubble through lime water
-
Lime water turns cloudy
-
-
-
Oxygen
-
Insert a glowing splint into a test tube
-
Splint relights
-
-
-
Hydrogen
-
Hold a lit splint at the mouth of a test tube
-
"Squeaky pop"
-
-
Flame tests (for soluble metal ions)
Flame tests test for metal (positive) ions in an ionic compound. This is the method:
- Clean a nichrome wire loop by submerging (completely) it in dilute hydrochloric acid.
- Dip the wire loop into the sample to be tested.
- Hold the wire loop in the blue flame of a Bunsen burner.
- Record the colour of the Bunsen flame.
Ion presentFlame test colour Lithium, Li+ Crimson Sodium, Na+ Yellow Potassium, K+ Lilac Calcium, Ca2+ Orange-red Copper, Cu2+ Green
Metal hydroxide precipitate tests
Dilute sodium hydroxide solution is used in tests for some metal ions, which form metal hydroxides that are insoluble. This means that the metal hydroxides appear as precipitates. For example, copper sulfate solution reacts with a few drops of sodium hydroxide solution:
Copper sulfate + Sodium hydroxide → Sodium sulfate + Copper hydroxide
CuSO4(aq) + 2NaOH(aq) → Na2SO4(aq) + Cu(OH)2(s)
Copper hydroxide forms a blue precipitate. Sodium hydroxide solution is added to copper sulfate solution. Solid copper hydroxide is produced in sodium sulfate solution
Metal hydroxide precipitate tests 2
Metal ion Precipitate colour
Aluminium, Al3+ White
Calcium, Ca2+ White
Magnesium, Mg2+ White
Copper(II), Cu2+ Blue
Iron(II), Fe2+ Green
Iron(III), Fe3+ Brown
Metal hydroxide precipitate tests 3
A few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. However, if excess sodium hydroxide solution is added:
- the aluminium hydroxide precipitate dissolves to form a colourless solution
- the calcium hydroxide precipitate is unchanged
- the magnesium hydroxide solution is unchanged
This means that using sodium hydroxide can give a positive result for aluminium ions, but it cannot distinguish between calcium and magnesium ions.
Ions of group 1 metals (Li+, Na+ and K+) form soluble hydroxides. Therefore, they are identified using flame tests and not by adding sodium hydroxide solution.
Negative ionic compound tests
Test for carbonate ions: CaCO3+2HCl→CaCl2+CO2+H2O.
The reaction of calcium carbonate with dilute hydrochloric acid yields calcium chloride (a salt), carbon dioxide, and water. Thus, to test for carbonate ions, add dilute HCl to the sample and the gas produced (CO2) if the sample contains carbonate ions should cause limewater to go cloudy
To test for sulfate ions:
- add a few drops of dilute hydrochloric acid to the sample.
- This removes carbonate ions. These could disrupt test results by forming a precipitate with the barium ions that are to be added
- add a few drop of dilute barium chloride solution
- A white precipitate forms if sulfate ions are present.
Ba2+(aq) + SO42-(aq) → BaSO4(s)
Halide tests
To test for halides, Silver ions react with halide ions (Cl-, Br- or I- ions) to form insoluble precipitates. To test for halide ions:
- add a few drops of dilute nitric acid to the sample
- add a few drop of dilute silver nitrate solution
The table shows the colours of these silver halide precipitates.
Halide ion Precipitate colour
Chloride, Cl- White
Bromide, Br- Cream
Iodide, I- Yellow
Reaction Profiles
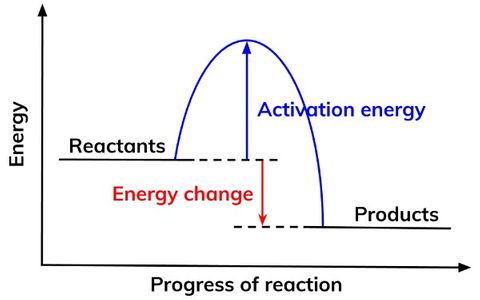
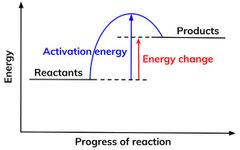
Reaction profiles show the progress of reaction on the x axis and energy level on the y axis.They contain the following information:
- The relative energies of reactants and products.
- The activation energy of a reaction.
- The overall energy change of a reaction.
Condensation polymerisation
%207.3.2.1%20-%20Condensation%20polymerisation%20of%20a%20diol%20and%20dicarboxylic%20acid-min,h_300,w_550.png)
- Condensation polymerisation describes the joining together of monomers with 2 functional groups to produce larger polymers, as well as small molecule by-products (e.g. H2O).
- The simplest condensation polymers are produced from 2 monomers with 2 of the same functional group on each monomer.
- E.g. ethanediol + hexanedioic acid → Terylene + water.
Atmosphere (present day)
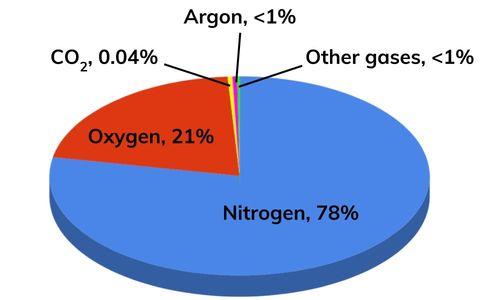
The proportions of gases in the Earth’s atmosphere has stayed more or less the same for the past 200 million years with rough values of:
- 78% nitrogen.
- 21% oxygen.
- Small quantities of other gases, including noble gases, carbon dioxide and water vapour. (1% remainder)
Atmosphere (Changes to present day)
Carbon dioxide
- For the first billion years of the Earth’s existence, highly active volcanoes populated the surface.
- The volcanoes frequently erupted. This released lots of carbon dioxide.
- Scientists think carbon dioxide dominated the early atmosphere in a similar way to modern-day Mars and Venus.
The volcanic activity also released:
- Nitrogen, which slowly built-up in the atmosphere.
- Water vapour, which condensed to form the oceans, as well as smaller quantities of methane and ammonia.
- The formation of oceans removed carbon dioxide from the atmosphere.
- Some of the dissolved carbon dioxide reacted with seawater to produce carbonate precipitates. These were deposited as sediment.
Atmosphere (Changes to present day)
Oxygen
- Algae were the first photosynthetic organisms to evolve approximately 2.7 billion years ago.
- Over the next billion years, photosynthesising plants began to evolve. This led to an ever-increasing rate of oxygen accumulating in the atmosphere, whilst removing carbon dioxide.
- Eventually, a threshold concentration of oxygen was reached which allowed more complex life forms to evolve, such as animals.
Greenhouse effect
%209.2.1.1%20-%20The%20greenhouse%20effect-min,h_300,w_550.png)
Greenhouse gases bring about the greenhouse effect. This helps keep the Earth warm enough for life. However, it can cause global warming when too strong. The process behind it is:
- The sun emits short wavelength infrared radiation that enters the atmosphere and travels towards the Earth’s surface.
- The Earth absorbs some of this radiation, but long wavelength radiation is reflected back into the atmosphere.
- Greenhouse gases can't absorb the frequency of radiation emitted by the Sun, but they can absorb the longer wavelength reflected radiation.
- The gases then re-radiate this energy in all directions, including back towards Earth, warming it globally.
Greenhouse effect 2
How humans increase the impact of the greenhouse effect:
- Agriculture (consatntly being increased) raises methane levels, due to lots of farm animals
- Landfills have mounds of waste are decomposing and decomposition releases methane.
- Burning fossil fuels releases levels of CO2 ( a greenhouse gas)
- Deforestation means less photosynthesis occurs so less CO2 is absorbed, meaning the greenhous effect can't be curbed as much since there is less photosynthesis
The current scientific consensus is that increased greenhouse gas emissions caused by changes to human activity will lead to global climate change (or already has).This consensus is based upon peer-reviewed evidence.
The global climate system is very complicated. This makes it difficult to create accurate models.Because of this, people speculate about climate change based on simpler models and inadequate (not enough or not reliable) information.
Some people may have reasons to play down the contribution of greenhouse gas emissions to climate change.If these biased opinions are published in the media, misinformation can spread.
Problematic pollutants
Incomplete combustion produces 2 problematic pollutants
- Carbon monoxide - a toxic gas
- Particulates - tiny particles of carbon & unburnt fuel (can cause respiratory issues and global dimmening)
Internal combustion engines in vehicules can cause nitrogen and oxygen to react (due to high temperatures whilst burning fossil fuels confined in a small space), which can lead to the formation of toxic nitrogen oxides, which can cause respiratory problems and acid rain (nitric acid)
Sulfur impurities (present in all fossil fuels) oxidises when burned creating sulfur dioxide, which can cause acid rain, which can cause corrosion (sulfuric acid)
Le Chatelier's principle (effect of temperature)
Change to Conditions and their Effect on closed system:
Decrease in temperature:
Changing the temperature for a reaction at equilibrium takes the system out of equilibrium. The system will react to try and restore the equilibrium.
The position of equilibrium will shift in the exothermic direction.This results in an increase in the products of the exothermic reaction and a decrease in the products of the endothermic reaction.
Increase temperature:
Changing the temperature for a reaction at equilibrium takes the system out of equilibrium. The system will react to try and restore the equilibrium.
The position of equilibrium will shift in the endothermic direction.This results in an increase in the products of the endothermic reaction and a decrease in the products of the exothermic reaction.
Le Chatelier's principle (effect of pressure)
Change to conditions and their effect on the closed system
Decrease in pressure:
Pressure changes only affect reactions involving gases. To predict what happens after a change in pressure, we must look at balanced equations to see how many gas molecules are on each side of the equation.
The position of equilibrium will shift towards the side of the reaction that produces the most gas molecules.
Increase in pressure:
Pressure changes only affect reactions involving gases. To predict what happens after a change in pressure, we must look at balanced equations to see how many gas molecules are on each side of the equation.
The position of equilibrium will shift to favour the reaction that produces the fewest gas molecules.
Le Chatelier's principle (effect of concentration)
Changes to conditions and their effect on the closed system:
Increase concentration of reactant / Decrease concentration of product:
Changing the concentration of any reactant or product takes the system out of equilibrium. The system will react to try to restore the equilibrium.
This will shift the position of equilibrium towards the products (forwards reaction).
Increase concentration of product / decrease concentration of reactants:
Changing the concentration of any reactant or product takes the system out of equilibrium. The system will react to try to restore the equilibrium.
This will shift the position of equilibrium towards the reactants (backwards reaction).
Water treatment
Water needs to be potable (safe to drink) so it needs to be treated to drastically reduce concentrations of microbes, dissolved minerals and salts found in the water.
Fresh water treatment:
1. Filtration: passed through wire mesh to remove solid particles.
2..Sterilisation: chlorine gas is added to kill harmful microorganisms.
Salt water treatment:
1. Desalination: There are two methods:
- Distillation: boiling seawater creates steam, which then condenses to give pure water.
- Reverse osmosis: seawater is passed through a selective membrane that only allows water molecules through.
Related discussions on The Student Room
- I enjoy chem practicals more than bio practicals. Is a chem degree right for me? »
- Is my degree unusually difficult or am I bust? »
- AS/A Level Chemistry Study Group 2023/2024 »
- How to increase grade in chemistry »
- Ask a Chemistry Uni Student! »
- physical or inorganic?? »
- Chemistry at Uni? »
- Bath Mpharm Interview Questions »
- OCR A Chemistry Predictions »
- Grade Growth Chronicles | From C's to A's (23-24) »


Comments
No comments have yet been made