Chemical and Energy Changes
- Chemistry
- Acids, bases and saltsElectrolysisEnergy of reactions/Exothermic and endothermic reactionsExtracting metals /The reactivity series
- GCSE
- AQA
- Created by: Maria is the best
- Created on: 08-02-18 19:38
Acids and Alkalis

Whether a substance is an acid/alkali depends on type of ions released when substance dissolved in water. Acids from hydrogen ions (H+) when dissolved in water and alkalis form hydroxide ions (OH-)
Neutralisation reactions
General equation for neutralisation reaction = acid + alkali --> salt + water
Neutralisation reactions between acids and alkalis can be shown in terms of H+ and OH- ions. H+ ions from acid react with OH- ions from alkali to produce water:
H+ + OH- --> H2O
Strong and Weak Acids
Acid Strength - tells you proportion of acid particles that'll dissociate to produce H+ ions in a solution.
Strong Acids - an acid (such as sulfuric, hydrochloric and nitrate acid) that completely dissociates its H+ ions in water (not a reversible reaction)
e.g. HCl(g) + H2O(l)→H+(aq) + Cl-(aq)
Weak Acids - an acid that only partially dissosiates its H+ ions in water (equilibrium-reversible reaction)
e.g. CH3COOH(aq)H+(aq) + CH3COO-(aq)
For every decrease of 1 on the pH scale the concentration of H+ ions increases by factor of 10. (x = difference in pH)
Factor H+ ion concentration changes by = 10-x
Reactions of Acids
A base is a substance that can accept a hydrogen ion (H+) from another substance. They're usally metal oxides or metal hydroxides.
Acid + Metal oxide/hydroxide --> salt + water
Metal carbonates are also bases - react with acids to produce carbon dioxide
Metal Carbonate + acid --> metal salt + carbon dioxide + water
Acid + Metal --> salt + hydrogen
Reactivity of Metals
Carbon and Hydrogen (non-metals) included in reactivity series as they're used to displace metals from their ores.
*The reactivity of metals is derived from how easily it loses an electron and becomes a postive ion
Reactivity of Metals...
Reactions that give evidence of reactivity = Metal + acid --> salt + hydrogen & Metal + water --> metal hydroxide + hydrogen
Displacement Reaction= Metal A + Metal B compound --> Metal B + Metal A compound (if A > B)
A more reactive metal will dispalce a less reactive metal from its compound. e.g. 2HCl + Mg --> MgCl2 + H2
Acids and Carbonates
*Most chlorides, sulphates + nitrates are soluble (can be dissolved)
*Most oxides, hydroxides + carbonates are insoluble (can't be dissolved)
Ca(OH)2(aq) + CO2(g) --> CaCO3(s) + H2O(l) ------ Precipitation reaction
*Precipitation reaction = when an insoluble solid is formed from a reaction
Precipitation reaction = (aq) + (aq) --> (s) or (g) + (aq) --> (s)
Redox Reactions
Oxidation can be defined as the gain of oxygen by an element or compound (1)
e.g. 2Mg + O2 --> 2MgO (*Mg has been oxidised - gained oxygen)
Reduction can be defined as the loss of oxygen by an element or compound (1)
e.g. 2CuO + C --> 2Cu + CO2 (*CuO has been reduced - lost oxygen)
Oxidation is the loss of electrons (2)
e.g. Zn(s) → Zn2+(aq) + 2e-
Reduction is the gain of electrons (2)
e.g. Cu2+(aq) + 2e-→ Cu(s)
Both oxidation and reduction happen at the same time - redox reaction - Cu2+(aq) + Zn(s)→Cu(s) + Zn2+(aq)
Electrolysis
'Electro' = electricity (molten)
'Lysis' = split (solution - dissolved)
IONIC - if solid (lattice) *no free ions to pass current (so has to be molten)
Electrolyte: a liquid/gel that contain ions and can be decomposed by electrolysis
Anode (+) --> attracts negative ions - Anions go to Anode
Cathode (-) --> attracts positive ions - Cations go to Cathode
Rules for predicting products at Electrolysis in solutions
- At negative electrode LEAST reactive ion loses electrons and forms an element
- At positive electrode if halides (group 7) present, halides will form
- At positive electrode if no halides present, OH- ions will produce O2
- At positive electrode if no halides or OH- ions present other negative ion will form its element
Energy transfer in reactions
Exothermic
- surroundings get 'hotter'
- combustion (CH4 + 2O2 --> CO2 + 2H2O)
- hand warmers
- neutralisation
Exothermic energy profile
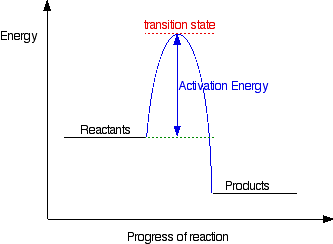
Energy transfer in reactions...
Endothermic
- suuroundings get 'colder' (loss of heat energy)
- ice packs
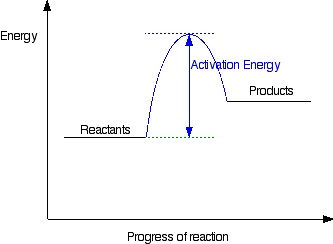
Energy transfer in reactions......
Activation Energy (Ea) = all reactions need a certain amount of energy to get started. This is called Activation Energy.
Endothermic breaks bonds so energy is absorbed
Exothermic makes bonds so energy is released
Heat: energy in a system (measured in Joules)
Temp: Average profile motion in a system (measured in Farenheit, Celsius, Kelvin - impossible to reache 0 in Kelvin as all particles have at least some kinetic energy.


Comments
Report
Report
Report
Report
Report