C4: Chemical Patterns
- Created by: emmacram
- Created on: 16-11-15 18:07
Development of the Periodic Table
- Elements are the 'building blocks' of all materials.
- The atoms of each element have a different proton number. The elements are arranged in order of ascending atomic (or proton) number, which gives repeating patterns in the properties of elements.
- Knowledge of chemical facts began to grow in the 18th and 19th centuries.
- Scientists tried to find patterns to stop themselves being overwhelmed by the mass of information and to provide a basis for understanding the facts.
- Many of the early attempts of classification were dismissed by the scientific community as more information emerged.
- Three of the most significant developments were made by Dobereiner, Newlands and Mendeleev.
- Dobereiner (1829) - arranged elements in groups of three, known as triads.
- The elements in each triad had similar chemical properties, and the relative atomic mass of the middle one was about halfway between the other two.
- Atomic mass at that time was measured relative to the mass of a hydrogen atom. It is now measured relative to 1/12 the mass of a carbon atom.
Development of the Periodic Table.
- John Newlands (1864) - Newlands only knew of the existence of 63 elements;many were still undiscovered.
- He arranged the known elements in order of relative atomic mass and found similar properties amongst every eighth element in the series.
- This makes sense since the noble gases (Group 0) weren't discovered until 1894.
- He had noticed some patterns, but the missing elements caused problems.
- His idea of putting elements into groups of eight had flaws and society greeted his idea with ridicule.
- The reasons for the flaws were later explained by the discovery of new elements, incorrect data and more complicated patterns after calcium.
- Dimitri Mendeleev (1869) - Mendeleev realised that some elements had yet to be discovered, so he left gaps to accommodate their eventual discovery.
- He used his periodic table to predict the existence of other elements.
- He also challenged some of the previous atomic mass data as being inaccurate.
Today's Periodic Table
Groups
- A vertical column of elements is called a group.
- Elements in the same group have the same number of electrons in their outer shell (except helium).
- This number also coincides with the group number.
- Group 1 elements have 1 electron in their outer shell and group 7 elements have 7.
- Elements in the same group have similar properties.
Periods
- A horizontal row of elements is called a period.
- The period to which an element belongs corresponds to the number of shells of electrons it has
- Sodium (Na) and Aluminium (Al) all have three shells of electrons and are in the third period.
- The periodic table can be used as a reference table to obtain important information about the elements.
- You can also tell if elements are metals or non-metals by looking at their position in the table. Metals are generally on the left side and non-metals on the right side of the table.
Atoms
- Chemists use atoms to explain the properties of the elements. All substances are made up of atoms (very small particles).
- Each atom has a small central nucleus, made up of protons and neutrons (with the exception of hydrogen), which is surrounded by electrons arranged in shells (or energy levels).
- An atom has the same number of protons as electrons, so the atom as a whole is neutral (has no electrical charge).
- A proton has the same mass as a neutron.
- The mass of an electron is negligible (nearly nothing, when compared to a proton or neutron).
- A substance that contains only one sort of atom is called an element.
- All atoms of the same element have the same number of protons.
- Atoms of different elements have different numbers of protons.
- The elements are arranged in the periodic table in order of increasing atomic (proton) number.
Spectroscopy
- When some elements are heated, they emit distinctive coloured flames.
- Lithium (red), sodium (yellow) and potassium (lilac) compounds can be recognised by the distinctive colours they produce in a flame test.
- The light emitted from the flame of an element produces a characteristic line spectrum.
- Each line in the spectrum represents an energy change as excited electrons fall from high energy levels to lower energy levels.
- The study of spectra has helped chemists to discover new elements.
- The discovery of some elements depended on the development of new practical techniques like spectroscopy.
- White light shows a continuous spectrum, but the spectrum of a lithium flame is made up of a series of lines.
Electron Configuration
- Electron configuration tells us how the electrons are arranged around the nucleus of an atom in shells (energy levels).
- The electrons in an atom occupy the lowest available shells (i.e. the shells closest to the nucleus).
- The first level (or shell) can only contain a maximum of two electrons.
- The second shell can hold a maximum of eight electrons.
- The third shell can hold up to 18 electrons (however, the last 10 of these electrons are only filled up after the first two of the fourth shell).
- The electron configuration is written as a series of numbers.
- There is a connection between the number of outer electrons and the group.
- You can also deduce the period to which an element belongs from its electron configuration.
- The chemical properties of an element are determined by its electon arrangement.
Hazards
Toxic- These substances can kill when swallowed, breathed in or absorbed through the skin.
Oxidising- These substances provide oxygen, which allows other substances to burn more fiercely.
Harmful- These substances are similar to toxic substances, but they are less dangerous.
Highly flammable- These substances will catch fire easily. They pose a serious fire risk.
Corrosive-These substances attack living tissue including eyes and skin and can damage materials
Explosive- These substances will explode when they are set alight.
Environmental hazard- These substances may present a danger to the environment.
Hazard Symbols
Safety Precautions
Some common safety precautions are:
- wearing gloves and eye protection, and washing hands after handling chemicals.
- using safety screens.
- using small amounts and low concentrations of the chemicals.
- working in a fume cupboard or ventilating the room.
- not eating or drinking when working with chemicals.
- not working near naked flames.
Group 1 - The Alkali Metals
- There are six metals in Group 1.
- As we go down the group, the alkali metals become more reactive.
- Alkali metals have low melting points.
- The melting and boiling points decrease as we go down the group.
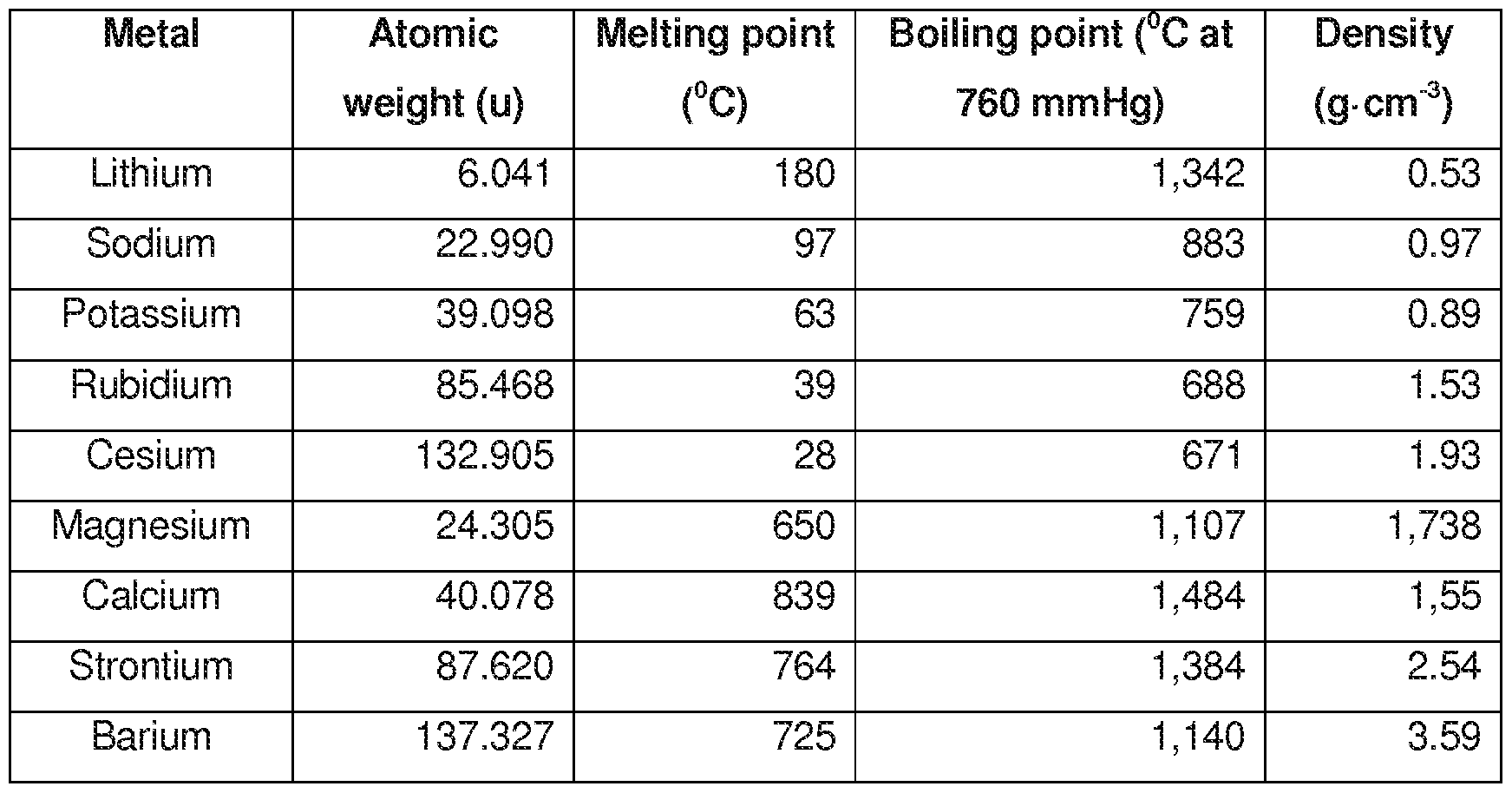
Alkali Metals
- When alkali metals react, they form compounds that are similar.
- The reaction becomes more vigorous as we go down the group because the metals are more reactive.
- Chlorine reacts vigorously with alkali metals to form colourless crystalline salts called metal chlorides.
- The alkali metals are stored under oil because they react very vigorously with oxygen and water. When freshly cut, they are shiny. However, they tarnish quickly in moist air, go dull and become covered in a layer of metal oxide.
- Lithium, sodium and potassium float on top of cold water (due to their low density). The heat from the reaction is great enough to turn sodium and potassium into liquids. Lithium reacts gently, sodium more aggressively and potassium so aggressively it catches fire.
- When alkali metals react with water, a metal hydroxide and hydrogen gas are formed. The metal hydroxide dissolves in water to form an alkaline solution.
Checking the pH Level
Follow these steps to check the pH level of the solution formed when an alkali metal is added to water:
- Put some universal indicator into a beaker containing water. The universal indicator will be green, indicating that the water is neutral (pH 7).
- Put a small piece of potassium into the beaker. It will react with the water and give off hydrogen gas.
- When it has finished reacting, the beaker will contain potassium hydroxide solution. The solution will now be purple, which indicates alkaline.
Group 1 Metals Precautions
When working with Group 1 metals, the following precautions should be taken:
- Use small amounts of very dilute concentrations.
- Wear safety glasses and use safety screens.
- Watch teacher demonstrations carefully.
- Avoid working near naked flames.
Group 7 - The Halogens
- There are five non-metals in group 7.
- At room temperature and room pressure, chlorine is a green gas, bromine is an orange liquid and iodine is a purple/dark grey solid.
- When heated, bromine forms a brown gas and iodine a pale purple gas.
- Chlorine is used to sterilise water and make pesticides and plastics.
- Iodine is used as an antiseptic to treat wounds.
- All halogens consist of diatomic molecules (they only exist in pairs of atoms). They can be used to bleach dyes and kill bacteria in water.
Group 7 Halogens Precautions
When working with halogens, the following precautions should be taken:
- Wear safety glasses.
- Work in a fume cupboard.
- Make sure the room is well ventilated.
- Use small amounts of very dilute concentrations,
- Avoid working near naked flames.
- Watch teacher demonstrations carefully.
Halogens
- A more reactive halogen will displace a less reactive halogen from an aqueous solution of its salt.
- Therefore, chlorine will displace both bromine and iodine, while bromine will displace iodine.
- When halogens react, they form compounds that are similar.
- The reactivity decreases as we go down the group.
- For example, this is seen clearly when halogens react with the alkali metals or iron.
- The reaction between chlorine and iron is more vigorous than that between iodine and iron.
Trends in Group 1 and Group 7
Group 1
- Alkali metals have similar properties because they have the same number of electrons in their outer shell, i.e. the highest occupied energy level contains one electron.
- The alkali metals become more reactive as we go down the group because the outer electron shell is further away from the influence of the nucleus and so an electron is lost more easily.
Group 7
- The halogens have similar properties because they have the same number of electrons in their outer shell, i.e. the highest occupied energy level contains seven electrons.
- The halogens become less reactive down the group because the outer electron shell is further away from the influence of the nucleus, so an electron is gained less easily.
Properties of Compounds
- Chemists use their observations to develop theories to explain the properties of different compounds.
- For example, experiments show that molten compounds of metals with non-metals, such as lithium chloride, conduct electricity.
- It can, therefore, be concluded that there must be charged particles in molten compounds. These particles are known as ions.
Ions
- If an atom loses or gains one or more electrons, it will carry an overall charge because the proton and electron numbers are no longer equal. When this happens, atom becomes an ion.
- If the ion has been formed by an atom losing electrons, it will have an overall positive (+) charge because it now has more protons than electrons. It is called a cation.
- If the ion has been formed by an atom gaining electrons, it will have an overall negative (-) charge because it now has more electrons than protons. It is called an anion.
- An ionic bond occurs between a metal and non-metal and involves the transfer of electrons from one atom to another to form electrically charged ions.
- Each electrically charged ion has a complete outer shell, i.e. the first shell has two electrons and each outer shell has eight electrons. Compounds of Group 1 metals and Group 7 elements are ionic compounds.
- Deducing the formula of an ionic compound - if you know the charge on both ions, you can work out the formula of an ionic compound.
- If you know the formula and the charge on one of the ions, you can work out the charge on the other ion.
- This is because all ionic compounds are neutral substances where the charge on the positive ion(s) is equal to the charge on the negative ion(s).
Related discussions on The Student Room
- Help chemistry urgent »
- Chem alevel help »
- Chemistry Alevel question »
- Chemical structures Biology A level »
- Please help me find marks (2 marks of an A* for A level chemistry) »
- Why does Scandium only exist in the 3+ ion »
- failing chemistry a-level, struggling to revise »
- Cambridge Chemical Engineering Interview »
- Best uni with cheap tuition for chemical engineering »
- chemistry as level equilibria help »
Comments
No comments have yet been made