Chemistry Unit 1
5.0 / 5 based on 3 ratings
- Created by: Lottie_C
- Created on: 29-03-16 12:22
atom
single particle of an element
1 of 77
compound
2 or more different atoms chemically joined (also a molecule)
2 of 77
molecule
2 or more atoms chemically joined
3 of 77
protons
positive charge, mass 1, nucleus, number of protons dictate what element it is
4 of 77
neutrons
no charge, mass 1, nucleus
5 of 77
electrons
negative, mass 1/2000, in shells/levels
6 of 77
what is the charge of an atom?
no overall charge as number of protons same as number of electrons
7 of 77
periodic table
each group has same amount of electrons in outer shell, similar properties
8 of 77
group 0
unreactive because stable arrangements of electrons
9 of 77
which elements are the most stable?
elements with a full outer shell
10 of 77
what does the number of electrons in the outer shell determine?
an element's chemical properties
11 of 77
conversation of mass
during chemical reactions atoms are neither created/destroyed, mass of products equals mass of reactants
12 of 77
mass number
sum of protons and neutrons
13 of 77
atomic number
number of protons
14 of 77
limestone
sedimentary rock, main mineral is calcium carbonate, often contains fossils
15 of 77
purest form of limestone
chalk
16 of 77
how is limestone formed?
chemical decomposition, accumulating corals or minute sea creatures shells
17 of 77
uses of limestone (9)
construction, building, toothpaste, glass, nutrient, neutralisation, cement, concrete, mortar
18 of 77
what is calcium hydroxide solution also known as?
limewater
19 of 77
what is the test for carbon dioxide?
It will turn limewater cloudy
20 of 77
What is calcium hydroxide also known as?
slaked lime
21 of 77
what is calcium oxide also known as?
quick lime
22 of 77
give an advantage and a disadvantage of quarrying
creates jobs, destroys habitats
23 of 77
thermal decomposition
carbonates of reactive metals will not decompose but most other do
24 of 77
oxidation
gaining of oxygen
25 of 77
reduction
loss of oxygen
26 of 77
general properties of metals
conduct heat and electricity, ductile, malleable
27 of 77
what is titanium's main ore?
rutile
28 of 77
what is aluminium's main ore and how is it extracted?
bauxite and electrolysis
29 of 77
ore
mineral with enough metal to make it worthwhile extracting
30 of 77
how can copper be extracted? (5)
smelting, bioleaching, electrolysis, chemical reaction, phytomining
31 of 77
Phytomining
uses plants to absorb metal compounds and the plants are burned to produce ash that contains metal compounds
32 of 77
bioleaching
uses bacteria to produce leachate solutions that contain metal compounds
33 of 77
electrolysis
positive ions move towards negative electrode
34 of 77
mineral
naturally occurring substances not part of a biological process
35 of 77
when can carbon be used as a reducing agent?
for metals less reactive than itself
36 of 77
2 advantages and 2 disadvantages of mining
creates jobs and wealth, environmental damage and waste
37 of 77
displacement
more reactive metal displaces a less reactive one from a compound
38 of 77
discovery of metals
1st metals discovered because they're naturally occurring and mostly unreactive
39 of 77
corrosion
protect metal by painting it to form protective oxide layer
40 of 77
alloys
addition of a few atoms of a different element which disrupts the layers so they can't slide over each other
41 of 77
recycling metals (3)
reduces landfill, reduces energy used, extracting them uses limited resources
42 of 77
Iron from a blast furnace
contains about 96% iron, brittle, limited uses, mostly converted to steel (alloy)
43 of 77
transition metals
centre block of periodic table, useful as structural materials
44 of 77
why is copper useful?
good conductor of electricity/heat (electrical wiring), can be bent but also hard (pipes or tanks), does not react with water (plumbing)
45 of 77
why are aluminium and titanium useful?
low density and resistance to corrosion
46 of 77
crude oil
mixture of many compounds containing hydrocarbons
47 of 77
hydrocarbons
molecules made up of hydrogens and carbons, carbon has 4 bonds, hydrogen has 1 bond
48 of 77
burning hydrocarbons
burn to make water and carbon dioxide and heat energy given off, if insufficient oxygen water and carbon monoxide given off
49 of 77
catalytic converter
carbon monoxide, nitric oxides, react over catalyst to form carbon dioxide and nitrogen
50 of 77
catalytic cracking
300-900 degrees celcius, zeolite catalyst/steam, no air, turns long chain alkanes to short chain alkanes/alkenes
51 of 77
test for alkene
few drops of bromine water added and it will turn colourless if its an alkene or stay brown if alkane
52 of 77
hydrogen
high energy fuel, large volume needed to store it
53 of 77
polymerisation
alkenes used for plastic (monomers), double bond opens up so they join together to form polymers
54 of 77
ethanol
made by fermentation of yeast (renewable) or hydration of ethene, lower energy content than petrol
55 of 77
pollution
burning fuels causes air pollution
56 of 77
Fractional distillation
separates hydrocarbons by using different boiling points in fractionating column, gets cooler as you go up the column
57 of 77
unsaturated oil
carbon-carbon double bonds, liquid
58 of 77
saturated fats
each carbon has 2 hydrogens, solid, molecules close together
59 of 77
hydrogenation
turning unsaturated fats to saturated fats, add hydrogen, 60 degrees celsius, nickel catalyst
60 of 77
plants
oil can be extracted from seeds/fruits, edible
61 of 77
benefits of oils
contain nutrients necessary to health, source of energy
62 of 77
emulsion
2 liquids which don't mix (immiscible) can have a substance added to stop them separating
63 of 77
emulsifier
special molecule with 2 ends, one which likes water and one which doesn't
64 of 77
olive oil
oil extracted by crushing olives and adding water, pressing the mixture, waiting for it to separate, then collecting oil
65 of 77
Tests for different fats
adding bromine/iodine to unsaturated oil will make a colourless solution. Adding bromine/iodine to saturated oil will make no change to colour
66 of 77
Distillation
steam generated and fed through mixture, oil from mixture evaporates and is carried through steam to condenser where it turns to a liquid
67 of 77
What did Wegener propose?
plate slowly moving, not believed because no evidence, not a geologist and couldn't explain theory
68 of 77
Evidence for plate tectonics
similar fossils found on different continents, similar rocks found on edges of different continents, edges of continents seem to fit together
69 of 77
movement of plates
generally slow, convection currents in mantle due to radioactive processes
70 of 77
earthquakes
difficult to predict as scientists don't know what's happening below crust and exactly how big/where/when
71 of 77
volcanoes
occurs when earth crust stretched thin and breaks to allow molten rock/magma to escape, seismometers predict tremors
72 of 77
Miller and Urey
primordial soup experiment, showed how molecules essential to life produce on Earth, other experiments like this, problems with it
73 of 77
Early atmosphere
major components were water vapour, carbon dioxide, minor components were methane and ammonia
74 of 77
today's atmosphere
nitrogen, oxygen, other gases, carbon dioxide
75 of 77
which planets have a similar atmosphere to Earth's early atmosphere?
Mars and Venus
76 of 77
Carbon Dioxide
oceans can absorb and release it, sea acts as reservoir for it, sedimentary rocks are a large source of it
77 of 77
Other cards in this set
Card 2
Front
compound
Back
2 or more different atoms chemically joined (also a molecule)
Card 3
Front
molecule
Back

Card 4
Front
protons
Back

Card 5
Front
neutrons
Back
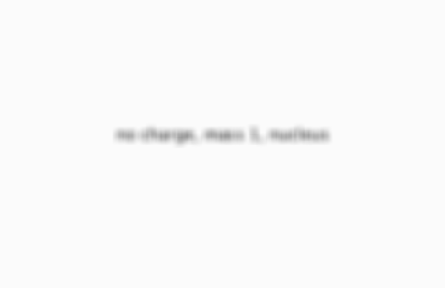
Related discussions on The Student Room
- Entry requirements university of Hertfordshire »
- How hard is the Btec level 3 extended certificate in applied science? »
- Is it possible to do the BTEC National Extended Certificate in one year? »
- BTEC Level 3 Applied Science Unit 1 May 2022 Exams »
- Applied Science Unit 1 Exam 2023 biology »
- Should I repeat Chemistry exams? »
- Edexcel chemistry unit 2 mixed questions »
- Official thread - January 2023 IAL edexcel »
- Bangor University GCSE Revision guides? »
- Help! Chemistry »
Similar Chemistry resources:
1.0 / 5 based on 1 rating
0.0 / 5
3.0 / 5 based on 1 rating
0.0 / 5
0.0 / 5
0.0 / 5
0.0 / 5
0.0 / 5
0.0 / 5
3.0 / 5 based on 1 rating


Comments
No comments have yet been made