Group 2, the alkaline earth metals
Teacher recommended
?- Created by: distortion
- Created on: 21-02-16 14:57
The group 2 metals are also known as the Alkaline earth metals. The group consists of the elements Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr) and Barium (Ba), decending in that order.
The metals in this group are considered 'S block' elements because their valance (outermost, bonding) electrons are located in s orbitals (subshells).
GROUP TRENDS IN PHYSICAL & CHEMICAL PROPERTIES
In general:
The trends for groups relate to the electron configuration of each of the elements.This means that from the previous element, as the nuclear charge increases - the electrons go into shells further from the nucleus.This creates extra distance of the outer shells from the nucleus and thus the electrostatic attraction between them and ultimately affects properties such as:
- The atomic radius - Ionic radius
- Ionisation energy - Melting points
- Chemical reactivity
ATOMIC RADIUS:
Trend down the group: Increases
Explaination: As you move down the group, each atom of each element has more electrons, therefore creating more electron shields.
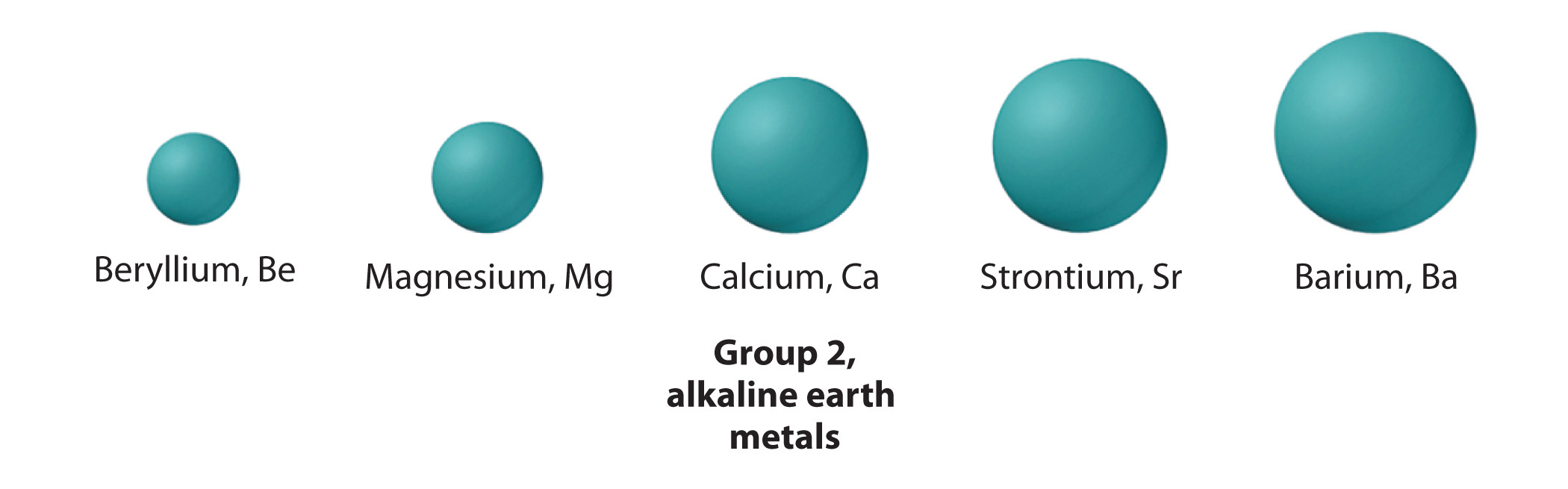
However, the atomic radius of group 2 elements decrease from their proceeding group 1 metals.
This is because as you move along the period, each element has an increase in nuclear charge of +1. This causes the electrostatic attraction between the nucleus and valence electrons to increase. With the same amount of sheilding, the stronger attraction draws the electron shells closer to the nucleus, therefore reducing the radius.
IONISATION ENERGY:
Trend down the group: Decreases
Explaination: The increase in electrons per atom of each element as you move down the group creates more electron sheilding between the nucleus and valence electrons. Increases sheilding therefore reduces the electrostatic attraction between these electrons and the nucleus. This means that less energy will be needed to remove a valence electron from the element as the force holding it there is weaker than in the elements higher up in the group.
Sucessive ionisation energies: increase
This could be due to the electron being removed coming from a higher energy subshell where less sheilding increases the attraction between the nucleus and the valence electrons and so more energy is required to overcome this attraction to remove another electron.
Also once…
Comments
Report
Report