Biological Molecules
- Created by: Gwen May Hutchings
- Created on: 14-11-18 10:11
Monosaccharides
They are made up of only one molecule. This molecule is made up of a chain of carbon atoms. Each carbon atom is connected to oxygen and hydrogen atoms in a certain way.
ALL Monosaccharides (and Disaccharides) are sweet and soluble.
ONLY Monosaccharides are small enough to pass across cell membrane.
Different Monosaccharides
Triose- Used in respiration and photosynthesis
Pentose- Used in DNA/RNA e.g. Ribose
Hexose- Bilogical Molecule e.g. Fructose, Galactose and Glucose
Isomers
- There is more than one molecule with the molecular formula C5H10O5 and more than one with the molecular formula C6H12O6. Molecules that have the same molecular formula but different structural formulae are called structural isomers.
Glucose
The small size and solubility in water of glucose molecules allow them to pass through the cell membrane into the cell. Energy is released when the molecules are metabolized. This is part of the process of respiration.
Two Glucose molecules join together via a condensation reaction, forming a disaccharide called Maltose.

Disaccharides
Maltose= Glucose + Glucose
Lactose= Glucose + Galactose
Sucrose= Glucose + Fructose

Benedict's Test for Reducing sugars
(All mono and disaccharides except sucrose)
- Add blue benedicts solution
- Heat
- Solution turns blue to pale green to yellow to orange to brick red- This is due to the precipitate copper oxide being formed
This is known as a semi-quantative test (The colour and density of the precipitate gives an indication of the amount of reducing sugar present, so this test is semi-quantitative)
Benedict's Test for Non-reducing sugars
(Sucrose)
- If Benedicts test is negative, add dilute acid
- Boil
- Neautralise with alkali
- Repeat Benedicts test
- Solution turns from blue to pale green to yellow to orange to brick red due to the precipitate copper oxide being formed.
Polysaccharides
Starch- Storage in plants
Glycogen- Storage in animals
Celulose- Structure of plants
Starch
- Energy storage product found in plant cells
- Insoluble-doesn't affect water potential of cells (osmosis)
- Cannot diffuse out of cells
- Compact- a lot can be stored in one place
- Easily hydrolyised to Glucose so it can be available quickly for transport or respiration
- Formed from alpha glucose molecules joined by glycosidic bonds.
- Mixture of two types of chains: Amylose and Amylopectin
Test for Starch
- Add yellow/brown iodine solution
- When starch is present the solution will turn a blue/black colour
- Qualitative test (shows whether a particular substance is present, but does not give an indication of how much is present)
Glycogen
- Energy storage product found in animal cells
- Found in animal cells- in skeletal muscles in the liver
Glycogen molecule:
- Highly branched chain of alpha glucose joined by 1,4 and 1,6 glycosidic links
- Uncoiled and each branch forms a 1,6 link
- Compact
Cellulose
- A structural carbohydrate- has a structural role in plants
- Provides rigidity to cell wall due to many weak hydrogen bonds having an overall strong effect
- Prevents cell from bursting when water enters by osmosis
Cellulose molecule:
- Unbranched long chais of beta glucose units joined by 1,4 glycosidic bonds
- Arrangement of 'right way up' followed by 'upside down' units enables Hydrogen bonds formation between adjacent chains.
Amino Acids
Proteins are polymers. The monomers of proteins are amino acids.
- All proteins have the same basic structure. They consist of an Amino Group at one end, an Acid Group at the other end, and a Carbon in the middle which bonds with a Hydrogen atom and an ‘R’ group, which is specific to individual amino acids.

Dipeptide bonds
When a peptide bond is formed by a condensation reaction between two amino acids, we get what is known as a dipeptide.

Formation of proteins
Primary Structure- Sequence of amino acids in the polypetide chain
Secondary structure- Where the polypeptide chains fold to form alpha helix and beta sheets due to hydrogen bonding
Tertiary Structure- Interaction of R groups causes irregular but specific folding of the polypeptide chains into globular structures. This is due to hydrophobic, hydrophillic, hydrogen, ionic and disulphide bonds.
Quaternery structure- More than 1 polypeptide chain
Lipids
- Contain carbon, hydrogen and oxgygen (unlike carbohydrates, lipids have a lot less oxygen)
- Non-polar molecules: Insoluble in water BUT soluble in organic solvents
- Hydrophobic
Types of Lipids
1. Triglycerides
2. Phospholipids
Triglycerides
Formed by the condensation reaction of 1 molecule of glycerol and 3 molecules of fatty acid.
- A condensation reaction between glycerol and a fatty acid = ester bond
- The hydrocarbon tail can be saturated or unsaturated and can also vary in length
- Hydrophobic and insoluble in water
Phospholipids
- In a Phospholipid, one of the fatty acids is substituted by a phosphate containing group.
- Phosphate head is polar (hydrophillic)
- Fatty acid tail is non polar (hydrophobic)
- Important in cell membrane structure

Test for Lipids: The Emulsion Test
1. Take a completely dry and grease-free test tube
2. To 2cm3 of the sample being tested, add 5cm3 of ethanol
3. Shake the tube thoroughly to dissolve and lipid in the sample
4. Add 5cm3 of water and shake gently
5. A cloudy-white colour indicates presence of a lipid
Cloudy colour is due to any lipid in the sample being finely dispersed in the water to form an emulsion. Light passing through this emulsion is refracted as it passes from oil droplets to water droplets makes it appear cloudy.
Enzymes
- All enzymes are globular proteins and biological catalysts
- They speed up reactions that would normally occur more slowly
- Work by lowering the activation energy that is needed for a reaction
- Specific- Active site is only complementary to a specific substrate
Lock and Key model

- Active site has rigid shape that is complementary to the substrate
- When they combine, they produce an enzyme-substrate complex
Induced fit model

- Enzyme has an active site similar to the shape of the substrate
- Collision = enzyme-substrate complex
- Slight change in shape of the active site causes strain on the 'transition state', weakening bonds
- Products are released leaving the active site unchanged and free to form another enzyme-substrate complex
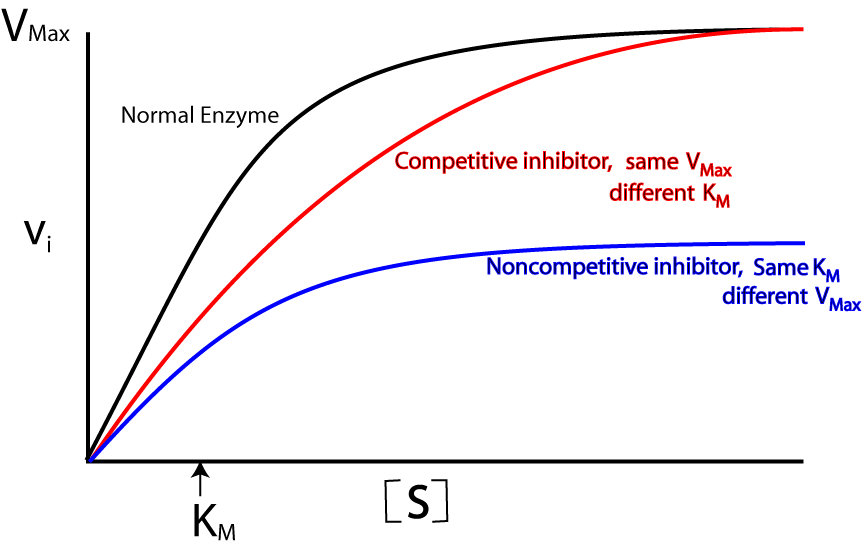
Enzyme Inhibition
An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. Inhibitors reduce the rate of reaction.
There are two types of inhibitors:
1. Competitive inhibitor
2. Non-competitive inhibitor
Competitive Inhibitor
- work by preventing the formation of Enzyme-Substrate Complexes because they have a similar shape to the substrate molecule
- This means that they fit into the Active Site, but remain unreacted since they have a different structure to the substrate.

Non-competitive inhibitor
- Non-competitive Enzyme Inhibitors work by preventing the formation of Enzyme-Product Complexes. So they prevent the substrate from reacting to form product.
- Doing so distorts the 3D Tertiary structure of the enzyme, therfore it can no longer catalyse a reaction.
Related discussions on The Student Room
- I enjoy chem practicals more than bio practicals. Is a chem degree right for me? »
- Biochemistry vs Chemistry vs Natural Sciences »
- Biochemistry at University »
- Any good youtube channels for Bio + Chem a levels? »
- Paper 3 AQA a Level biology »
- Access to Science course »
- 25 mark essay question »
- Biochemistry Personal Statement Example »
- Whats beter revising a whole topic for 1 subject or 2 lessons for 2 subjects A level »
- Do I need to know how to draw structures for carbohydrates? (AQA A Level Bio) »
Comments
No comments have yet been made