Module C1- Carbon Chemistry
- Created by: KatrinaAnnMarie
- Created on: 31-03-15 08:49
Atoms, molecules and compounds...
Atoms have a positive nucleus with orbiting electrons
Atoms have a positively charged nucleus and electrons which are negatively charged. The electrons move around the nucleus in layers known as shells.
Atoms can form bonds to make molecules or compounds. It's electrons that are involved in making bonds. Sometimes an atom loses or gains one or more electrons and this gives it a charge, (positive if it loses an electron and negative if it gains one).
Charged atoms are known as ions. If a positive ion meets a negative ion they'll be attracted to each other and join together. This is called an ionic bond. The other main type of bond is called a covalent bond--where atoms share a pair of electrons.
Additives in food
Additives
Additives are added to lots of our foods to improve their flavour, colour or to make them last longer.
- Food colours--makes it look more appestising
- Flavour enhancers--bring out the taste and smell of food without adding taste of their own
- Antioxidants--help to preserve food
- Emulsifiers--help oil and water blend together in foods such as salad cream and ice-cream
Emulsifiers
What is an emulsifier?
- You can mix an oil with water to make an emulsion
- Emulsions are made up of lots of droplets of one liquid suspended in another liquid
- Oil and water naturally separate into two layers with the oil floating on top of the water--they don't "want" to mix. Emulsifiers help to stop the two liquids in an emulsion from separating
- Emulsifiers are molecules with one part that's attracted to water and another part that's attracted to oil or fat
- The part attracted to water is called--hydrophilic
- Thepart attracted to oil is called--hydrophobic
Hydrophilic and hydrophobic...
Hydrophilic
The hydrophilic "head" is attracted to water molecules. This end of the emulsifier molecule bonds to water molecules.
Hydrophobic
The hydrophobic "tail" is attracted to oil molecules. This end of the emulsifier molecule bonds to oil molecules.
Cooking and chemical change
Cooking food produces new substances. That means a chemical change has taken place. Once cooked, you cannot change it back. Cooking is an example of an irreversible reaction.
Eggs and meat are good sources of protien. Protien molecules change shape when you heat them. The energy from cooking breaks some of the chemical bonds in the protien this allows the molecule to take a different shape. This gives the food a more edible texture. The change is irreversible and it's called denaturing.
Potatoes are plants, so each potato cell is surrounded by a rigid cell wall made of cellulose. Humans can't digest cellulose. Cooking the potato ruptures (breaks down) the cell walls. It also makes the starch grains inside the cells swell up and spread out. These changes make the potato softer and more flexible, and much easier to digest.
Baking powder and heating
When you heat baking powder, it undergoes thermal decomposition.
Thermal decomposition is when a substance breaks down into simpler substances when heated. Many thermal decompositions are helped along by a catalyst. (Thermal decomposition is different from a lot of reactions you'll come across, since there's only one substance to start with).
Baking powder contains the chemical sodium hydrogencarbonate. The symbol equation is:
Baking powder is used in baking cakes--the carbon dioxide produced makes the cakes rise.
You can check that it is actually carbon dioxide that has been formed by using a chemical test:
Carbon dioxide can be detected using limewater--CO2 turns the limewater cloudy.
Perfumes
Chemicals that smell nice are used as perfumes and air freshners. Esters are often used as perfumes as they usually smell quite pleasant.
Esters are pretty common in nature. Loads of common fruity smells and flowery smells contain natural esters.
Esters can be manufactured synthetically to use as perfumes or flavourings, e.g. there are esters (or combinations of esters) that smell of lavendar, oranges, cinnamon, etc.
Making esters
You can make an ester by heating a carboxylic acid with an alcohol. (This is an example of esterfication).
An acid catalyst is usually used e.g. concentrated sulfuric acid:
alcohol + organic acid → ester + water
For example - methanol + butanoic acid → methyl butanoate + water
Properties of perfumes
You can't use any old chemical with a smell as a perfume. You need a substance with certain properties:
Easily evaporates--or else the perfume particles won't reach your nose and you won't be able to smell it...pointless
Non-toxic--it cannot poison you
Doesn't react with water--or else it would react with water in sweat
Doesn't irritate your skin--or else you couldn't apply it directly to your skin. You could risk burning youself
Insoluble in water--if it was soluble in water it would wash off every time you got wet
Properties of solids
- There are strong forces of attraction between particles, which holds them in fixed positions in a very regular lattice arrangement
- The particles don't move from their positions, so all solids keep a definate shape and volume, and don't flow like liquids
- The particles vibrate about their positions--the hotter the soild becomes, the more they vibrate (causing solids to expand slightly when heated)
If you heat the solid (give the particles more energy), eventually the solid will melt and beome a liquid.
Properties of liquids
- There is some force of attraction between the particles. They're free to move past each other, but they do tend to stick together
- Liquids don't keep a definate shape and will flow to fill the bottom of a container. But they do keep the same volume
- The particles are constantly moving with random motion. The hotter the liquid gets, the faster they move. This causes liquids to expand slightly when heated
If you heat the liquid, eventually it will boil and become a gas.
Properties of gases
- There's next to no force of attraction between the particles--they're free to move. They travel in straight lines and only interact when they collide
- Gases don't keep a definate shape or volume and will always fill any container. When the particles bounce off the walls of a container they exert a pressure on the walls
- The particles move constantly with random motion. The hotter the gas gets, the faster they move. Gases either expand when heated, or their pressure increases
How we smell...volitatlity is key.
- When a liquid is heated, the energy goes to the particles, which makes them move faster
- Some particles move faster than others.
- Fast-moving particles at the surface will overcome the forces of attraction from the other particles and escape. This is evaporation.
- How easily a liquid evaporates is called its volatility.
The evaporated particles drift in the air and the smell receptors in your nose pick up the chemicals. Perfumes are quite volatile so that they can evaporate enough for you to smell them. The particles in liquid perfumes only have a very weak attraction between them...so you only need a very small amount of heat energy to make them evaporate.
Solutions
A solution is a mixture of a solvent and solute.
When you add a solid (a solute) to a liquid (a solvent) the bonds holding the solute molecules together sometimes break and the molecules then mix with the molecules in the liquid--forming a solution. This is called dissolving.
Whether the bonds break depends on how strong the attractions are between the molecules within each substance and how strong the attractions are between the two substances.
Key Definitions
Solution--is a mixture of a solute and a solvent that does not separate out.
Solute--is the substance being dissolved.
Solvent--is the liquid it's dissolving into.
Soluble--means it will dissolve.
Insoluble--means it will not dissolve.
Solubility--a measure of how much will dissolve.
Paints
Paints usually contain: solvent, binding medium and pigment.
- The pigment gives the paint its colour
- The binding medium is a liquid that carries the pigment bits and holds them together. When the binding medium goes solid it sticks the pigment to the surface you've painted
- The solvent is the stuff that thins the paint and makes it easier to spread
Paints are colloids
- A colloid is consists of really tiny particles of one kind of stuff dispersed in (mixed with) another kind of stuff. They're mixed in, but not dissolved
- The particles can be bits of solid, droplets of liquid or bubbles of gas
- Colloids don't separate out because the particles are so small. They don't settle out at the bottom
- A paint is a colloid where particles of a pigment (usually a solid) are dispersed through a liquid.
Emulsion paints
- Emulsion paints are water-based. The solvent used in these paints is water, and the binding medium is usually an acryllic or vinyl acetate polymer
- A water-based emulsion dries when the solvent evaporates, leaving behind the binder and pigment as a thin solid film. A thin layer of emulsion paint dries quite quickly.
- Emulsion paints are fast-drying and don't produce harmful fumes--so they're ideal for painting things like inside walls.
Traditional gloss paints
- Traditional gloss paints and artists' oil paints are oil-based. This time, the binding material is oil, and the solvent is an organic compound that'll dissolve in oil.
- Oil paints dry in two stages. First the solvent evaporates, and then the oil is oxidised by oxygen in the air before it turns solid. (They tend to take longer to dry than water-based paints).
- Oil paints are glossy, waterproof and hard-wearing, but the solvents used to make them often produce harmful fumes. They're best used for painting things like, outside doors and metalwork.
Thermochromic pigments
Thermochromic pigments change colour when heated.
- Thermochromic pigments change colour or become transparent when heated or cooled
- Different pigments change colour at different temperatures, so a mixture of different pigments can be used to make a colour-coded temperature code. These are used to make basic thermometers that you stick on your forehead to take your temperature.
Thermochromic pigments can be mixed with acrylic paint, giving a wide range of colour changes. For example, mixing a blue thermochromic pigment that loses its colour about 27C, with yelloq acrylic paint would give paint that's green below 27C and yellow about 27C.
These paints are used on novelty mugs. For example, some mugs have a design that changes colour when a hot drink's poured into them. Other mugs use thermochromic pigment that beomes transparent when heated. A picture beneath the paint is only visible when a hot drink is poured in.
Phosphorescent pigments
- Phosphorescent pigments absorb natural or artificial light and store the energy in their molecules. This energy is released as light over a period of time--from a few seconds to a couple of hours.
- An abvious use is a watch or clock with glow-in-the-dark hands.
- Other uses include traffic signs, emergency exit signs, toys and novelty decorations.
- Glow-in-the-dark watches used to be made with radioactive paints. These paints would glow for years without needing to be "charged up". Unfortunately, a lot of them weren't safe and could give quite a dose of atomic radiation. Phosphorescent were developed as a much safer alternative.
Polymers
Plastics are long-chain molecules called polymers:
- Polymers are formed when lots of small molecules called monomers join together. This reaction is called polymerisation--and it usually needs high pressure and a catalyst.
- Plastics are polymers. They're ususally carbon based and their monomers are alkenes.
Addition Polymers:
- The monomers that make up addition polymers have a double covalent bond.
- Molecules with at least one double covalent bond between carbon atoms are called unsaturated compounds. Molecules with no double bond between carbon atoms are called saturated compounds.
- Lots of unsaturated monomer molecules (alkenes) can open up their double bond and join together to form polymer chains.
Forces between molecules determine properties of p
- Stong covalent bonds hold the atoms together in polymer chains. But it's the forces between the different chains that determine the properties of the plastic.
Weak forces:
If the plastic is made up of long chains that are held together by weak intermolecular forces, then the chains will be free to slide over each other. This means that the plastic can be stretched easily, and will have a low melting point.
Strong forces:
Some plastics have stronger bonds between the polymer chains--these might be covalent bonds between the chains, or cross-linking bridges. These plastics have higher melting points, are rigid and can't be stretched, as the crosslinks hold the chains firmly together.
Polymers
Different polymers have different physical properties--some are stronger, some are stechier, some are more easily moulded, and so on. These different physical properties make them suited for different uses.
- Strong, rigid polymers such as high density polythens are used to make plastic milk bottles.
- Light, stretchable polymers such as low density polyethene are used for plastic bags and squeezy bottles. Low density polyethene has a low melting point, so it's no good for anything that'll get very hot.
- PVC is strong and durable, and it can be made either rigid or stretchy. The rigid kind is used to make window frames and piping. The stretchy kind is used to make synthetic leather
- Polystyrene foam is used in packaging to protect breakable things, and it's used to make disposable coffee cups (the trapped air in the foam makes it a brilliant thermal insulator).
Fractional distillation
- Crude oil is formed from the buried remains of plants and animals--it's a fossil fuel. Over millions of years, with high temperature and pressure, the remains turn to crude oil, which can be drilled up.
- Crude oil is a mixture of lots of different hydrocarbons. Remember that hydrocarbons are chains of carbon atoms (e.g. alkanes and alkenes) of various lengths.
- The different compounds in crude oil are separated by fractional distillation. The oil is heated until most of it has turned into gas. The gases enter a fractionising column (and the liquid bit, bitumen, is drained off at the bottom). In the column there's a temperature gradient (i.e. it's hot at the bottom and gets gradually cooler as you go up).
- The longer hydrocarbons have high boiling points. The shorter hydrocarbons have lower boiling points. They turn to liquid and drain out much later on, near to the top of the column where it's cooler.
- You end up with the crude oil mixture separated out into different fractions. Each fraction contains a mixture of hydrocarbons with similar boiling points.
Hydrocarbon properties
As the size of the hydrocarbon molecule increases:
- The boiling point increases
- It gets less flammable
- It gets more viscous
- It gets less volatile
That's how fractional distillation works--you can separate out the random mixture of all kinds of hydrocarbons into groups (fractions) that have similar chain lengths and so similar properties. Then, you can use them for various useful things like powering vehicles, heating homes and making roads. It works because one of those properties that each group has in common is the boiling point.
Cracking
Cracking is splitting up long-chain hydrocarbons:
- Cracking turns long alkane molecules into smaller alkane and alkene molecules (which are much more useful).
- It's a form of thermal decomposition, which is when one substance breaks down into at least two new ones when you heat it. This means breaking strong covalent bonds, so you need lots of heat and a catalyst.
- A lot of the longer molecules produced from fractional distillation are cracked into smaller because there's more demand for products like petrol and kerosene (jet fuel) than for diesel or lubricating oil.
- Cracking also produced lots of alkene molecules, which can be used to make polymers (mostly plastics)
Complete combustion
Complete combustion happens when there's plenty of oxygen. The complete combustion of any hydrocarbon in oxygen will produce only carbon dioxide and water as waste products, which are both quite clean and non-poisonous.
- Many gas heaters release these waste gases into the room, which is perfectly ok. As long as the gas heater is working properly and the room is well ventilated there's no problem.
- This reaction, when there's plenty of oxygen. It is known as complete combustion. It releases lots of energy and only produces those two harmless waste products.
- When there's plenty of oxygen and combustion is complete, the gas burns with a clean blue flame.
- You need to be able to give a balanced symbol equation for the complete combustion of a simple hydrocarbon fuel when you're given its molecular formula.
Incomplete combustion
Incomplete combustion of hydrocarbons is not safe.
- If there isn't enough oxygen the combustion will be incomplete. This gives carbon monoxide and carbon as waste products, and produces a smoky yellow flame.
- The carbon monoxide is colourless, odourless and a poisonous gas and it's very dangerous.
- You want lots of oxygen when you're burning fuel--you get more heat energy given out, and you don't get any messy soot or poisonous gases.
You need to be able to write a balanced symbol equation for incomplete combustion:
hydrocarbon + oxygen → carbon monoxide + carbon + water
CH4 + 1.5 O2 → CO + 2 H2O
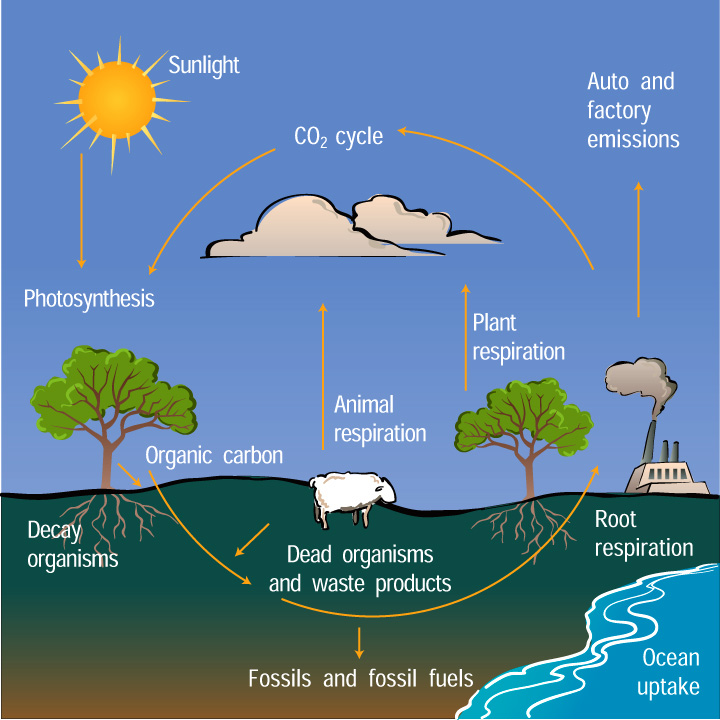
The carbon cycle
Air pollution and acid rain.
Acid rain is caused by sulfur dioxide and oxides of nitrogen:
- When fossil fuels are burned they release mostly CO2 (a big cause of global warming).
- But they also release other harmful gases--especially sulfur dioxide and various nitrogen oxides.
- The sulfur dioxide (SO2) comes from sulfur impurities in the fossil fuels.
- However, the nitrogen oxides are created from a reaction between the nitrogen and oxygen in the air, caused by the heat of the burning. (This can happen in the internal combustion engines of cars).
- When these gases mix with clouds they form dilute sulfuric acid and dilute nitric acid.
- This then falls as acid rain.
- Power stations and internal combustion engines in cars are the main causes of acid rain.
Acid rain
- Acid rain causes lakes to become acidic and many plants and animals die as a result
- Acid rain kills trees and damages limestone buildings and ruins stone statues. It also makes metal corrode.
Carbon monoxide
Carbon monoxide is a poisonous gas:
- Carbon monoxide (CO) can stop your blood doing its proper job of carrying oxygen around the body.
- A lack of oxygen in the blood can lead to fainting, a coma or death.
- Carbon monoxide is formed when petrol or diesel in car engines is burnt without enough oxygen--this is incomplete combustion.
Air pollution and acid rain.
It's important that atmospheric pollution is controlled:
- The build-up of all these pollutants can make life unhealthy and miserable for many humans, animals and plants. The number of cases of respiratory illnesses (e.g. asthma) has increased in recent years--especially among young people. Many people blame atmospheric pollution for this, so efforts are being made to improve things.
- Catalytic converters on motor vehicles reduce the amound of carbon monoxide and nitrogen oxides getting into the atmosphere. The catalyst is normally a mixture of platinum and rhodium.
It helps unpleasant exhaust gases from the car react to make things that are less immediately dangerous (though more CO2 is still not ideal).


Comments
No comments have yet been made