Chapter 3 ~ Biological Molecules
- Created by: SXGXNXX27
- Created on: 27-05-19 22:11
Roles of Cations
Calcium ions (Ca2+): necessary for nerve impulse transmission and muscle contraction
Sodium ions (Na+): necessary for nerve impulse transmission and kidney function
Potassium ions (K+): necessary for nerve impulse transmission and stomatal opening
Hydrogen ions (H+): necessary for catalysis of reactions and pH determination
Ammonium ions (NH4+): necessary for production of nitrate ions by bacteria
Roles of Anions
Nitrate ions (NO3-): nitrogen supply to plants for amino acid and protein formation
Hydrogen carbonate ions (HCO3-): maintenance of blood pH
chloride ions (Cl-): balance positive charge of potassium and sodium ions in cells
Phosphate ions (PO4 3-): cell membrane formation, nucleic acid and ATP formation and bone formation
Hydroxide ions (OH-): catalysis of reactions and pH determination
Biological Molecules
Here is a summary of the elements in some of the key biological molecules:
Carbohydrates: carbon, hydrogen and oxygen (usually in the ratio Cx(H2O)x
Lipids: carbon, hydrogen and oxygen
Proteins: carbon, hydrogen, oxygen, nitrogen and sulfur
Nucleic acids: carbon, hydrogen, oxygen, nitrogen and phosphorus
- Biological molecules are often polymers
- Polymers are long chain molecules made up by the linking of individual molecules called monomers in a repeating pattern
- In carbohydrates, the monomers are sugars (saccharides)
- In proteins, the monomers are amino acids
Characteristics of Water
Unusually high boiling point
- The hydrogen bonds between molecules require a lot of energy to break.
Ice is less dense than liquid water
- As water cools below 4*c, the hydrogen bonds fix the positions of the polar molecules slightly further apart than the average distance in the liquid state. This produces a giant rigid but open structure with every oxygen atom at the centre of a tetrahedral arrangement of hydrogen atoms. Therefore, ice floats on water.
Cohesive and adhesvive properties
- Water molecules move as one mass as the molecules are attracted to each other (cohesion).
- Water molecules are attracted to other materials (e.g when you wash your hands, they get wet; the water doesn't run straight off).
Water for Life
- As water is a polar molecule, it acts as a solvent in which many of the solutes in an organism can be dissolved. Water acts as a medium for chemical reactions and helps transport dissolved compounds into and out of cells.
- Water makes a very efficient transport medium in living things.The effects of cohesion and adhesion results in water exhibiting capillary action - the process by which water can rise up a narrow tube against the force of gravity.
- Water acts as a coolant helping to buffer temperature changes during chemical reactions in prokaryotic and eukaryotic cells because of the large amounts of energy required to overcome hydrogen bonding. (Maintaining a constant temperatures in cellular environments is important as enzymes are often only active in a narrow temperature range).
- Water is stable (does not change temp or become a gas easily) therefore providing a constant environment. As ice floats, it forms an insulating above for the water below. Some organisms also inhabit the surface of water, as surface tension is strong enough to support small insects.
Carbohydrates Introduction
- They're also known as saccharides or sugars
- A single sugar unit is known as a monosaccharide (e.g. glucose, fructose and ribose)
- When two monosaccharides link together they form a disaccharide (e.g. maltose, lactose and sucrose)
- When two or more monosaccharides are linked together they form a polymer called a polysaccharide (e.g. glycogen, cellulose and starch)
Glucose
- Chemical formula: C6H12O6
- It's a monosaccharide composed of six carbons, therefore it's a hexose monosaccharide.
- There are two structural variations of the glucose molecule (alpha and beta)

- Glucose molecules are polar and soluble in water - due to the hydrogen bonds that form between the hydroxyl groups and water molecules.
- Its solubility in water means that glucose is dissolved in the cytosol of the cell.
Condensation Reactions
- When to alpha glusoce molecules are side by side, the hydroxyl groups interact.
- Two hydrogen atoms and one oxygen atom are removed from the glucose monomers and join to form water.
- A bond (1,4 glycosidic bond) forms between carbon 1 and carbon 4 on the glucose molecules.
- In this reaction, the new molecule (disaccharide) is called maltose.
- Fructose and galactose are also hexose monosaccharides.
- Glucose + Glucose -----> Maltose
- Glucose + Fructose -----> Sucrose
- Glucose + Galactose -----> Lactose
- Pentose monsaccharides are sugars that contain five carbon atoms.
- Ribose is the pentose sugar present in RNA nucleotides.
- Deoxyribose is the pentose sugar present in DNA nucleotides.
Starch
Many alpha glucose molecules can be joined by glycosidic bonds to form two slightly different polysaccharides known collectively as starch. Glucose made by photosynthesis in plant cells is stored as starch. It is a chemical energy store.
- One of the polysaccharides in starch is called amylose. Formed by alpha glucose molecules joined together by only 1,4 glycosidic bonds. The angle of the bond means that the long chain of glucose twists to form a helix which is further stabilised by hydrogen bonding within the molecule. This makes the polysaccharide more compact and much less soluble than the glucose molecules used to make it.
- The other starch polysaccharide is called amylopectin. It's also made by 1,4 glycosidicbonds, bu there are also 1,6 glycosidic bonds occuring approximately once in every 25 glucose subunits. Therefore, amylopectin has a branched structure.
Glycogen
Glycogen is the functionally equivalent energy storage molecule to starch in animals and fungi.
- Glycogen forms more branches than amylopectin, which means it is more compact and less space is needed for it to be stored.
- This is important as animals are mobile unlike plants.
- The coiling or branching of these polysaccharides makes them very compact, which is ideal for storage.
- The branching also means that there are many free ends where glucose molecules can be added or removed.
- This speeds up the processes of storing or releasing glucose molecules required by the cell.
Hydrolysis Reactions
- Glucose is stored as starch by plants or as glycogen by animals and fungi until it is needed for respiration - the process in which biochemical energy in these stored nutrients is converted into a useable energy source for the cell.
- To release glucose for respiration, starch or glycogen undergo hydrolysis reactions.
- This requires the addition of water molecules and the reactions are catalysed by enzymes.
- These are the reverse of the condensation reactions that form the glycosidic bonds.

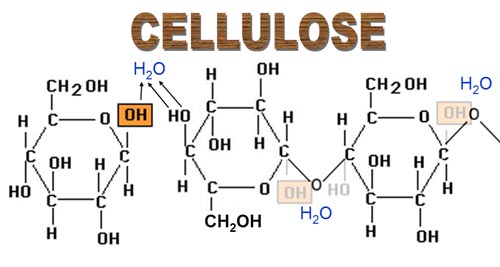
Cellulose
- Beta glucose molecules are unable to join together in the same way as alpha glucose molecules.The hydroxyl groups on carbon 1 and carbon 4 are too far apart from each other to react.
- The only way that beta glucose molecules can join together, is if the alternate glucose molecules are turned upside down.
- The structure is unable to coil or form branches, therefore a straight chain polysaccharide is formed called cellulose.
- Cellulose molecules form hydrogen bonds with each other forming microfibrils. These microfibrils join together forming macrofibrils, which combine to produce fibres.
- These fibres are strong and insoluble and are used to make cell walls.
- Cellulose is an important part of our diet, it's very hard to break the bonds and form monomers. It forms the 'fibre' or 'roughage' necessary for a healthy digestive system.
Benedict's Test for Reducing & Non-Reducing Sugars
Reduction is the reaction involving the gain of electrons. All monosaccharids and some disaccharides (e.g. maltose and lactose) are reducing sugars. This means that they can donate electrons or reduce another chemical or molecule. [Benedict's reagent is an alkaline solution of copper(II) sulfate].
1) Place the sample to be tested in a boiling tube. if it is not in liquid form, grind it up or blend it in water.
2) Add and equal volume of Benedict's reagent.
3) Heat the mixture gently in a boiling water bath for 5 minutes.
Reducing sugars will react with the copper ions in Benedict's reagent. This results in the addition of electrons to the blue Cu2+ ions, reducing them to brick red Cu+ ions. The formation of a brick red precipitate indicates a positive test result.
Non-reducing sugars (e.g. sucros) will not react with the solution and therefore the solution will remain blue indicating a negative test result. If sucrose is fist boiled with dilute HCl, the test will be positive as sucrose has been hydrolysed by the acid to form fructose and glucose.
Iodine Test & Reagent Strips
Iodine test
- used to detect presence of starch
- few drops of iodine dissolvedin potassium iodide solution are mixed with a sample
- if the solution changes colour from yellow/brown to purple/black, starch is present in the sample.
- if the iodine solution remains yellow/brown, it is a negative result and starch is not present
Reagent strips
- manufactured reagent strips can be used to test for the presence of reducing sugars, most commonly glucose
- with a colour-coded chart, the concentration of sugar can be determined
Colorimetry
A colorimeter is piece of equipment used to quantitatively measure the absorbance or transmission of light by a coloured solution. The more concentrate a solution is, the more light it will absorb and the less light it will transmit. this can be used to calculate the concentration of reducing sugar present.
- A filter is placed in the colorimeter.
- Calibrate the colorimeter with distilled water.
- Perform the Benedict's Test on the solutions.
- Filter the resulting solutions to remove the precipitate.
- Work out the % transmission (% absroption = 100% - % transmission).
- A callibration curve can be plotted if a range of different solutions with known concentrations (of e.g. glucose) were tested.
- This can then be used to work unknown concentrations.
Biosensors
Biosensors use biological components to determine the presence and concentrations of molecules such as glucose. (Analyte - compound under investigation).
- Molecular recognition - a protein (enzyme or antibody) or single strand of DNA (ssDNA) is immobillised to a surface (e.g. a glucose test *****). This will interact with, or bind to, the specific molecule under investigation.
- Transduction - this interaction will cause a change in a transducer. A transducer detects changes, for example in pH, and produces a response such as a the release of an immobilised dye on a test ***** or an electric current in a glucose-testing machine.
- Display - this then produces a visible, qualitative or quantitative signal such as a partcular colour on a test ***** or a reading on a test machine.
Lipids Introduction
- Commonly known as fats (solid at room temp) and oils (liquid at room temp)
- Non-polar molecules, so are isoluble in water
- Lipids are large molecules known as macromolecules - not built from repeating units or monomers like polysaccharides
Triglycerides
- Made by combining one glycerol molecule with three fatty acids
- Glycerol is a member of the alcohols
- Fatty acids are a member of the carboxylic acids
- Both molecules contain a hydroxyl group. These hydroxyl groups react to form three water molecules in a condensation reaction called esterification (as ester bonds are formed)
- When triglycerides are broken down, three water molecules need to be supplied to reverse the reaction that formed the triglyceride - this is also a hydrolysis reaction.

Saturated & Unsaturated Triglycerides
Saturated
- No double bonds between carbon atoms
Monounsaturated
- Only one double bond present
Polyunsaturated
- Two or more double bonds present
Presence of double bonds causes molecule to bend which means they cannont pack so closely together. This maked them liquid at room temperature (known as oils).
Plants contain unsaturated triglycerides, which normally occur as oils, and tend to be more healthy in the diet than saturated triglycerides.
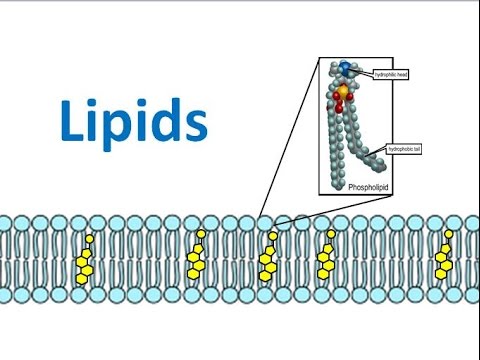
Phospholipids
- They're modified triglycerides
- Contain the element phosphorus along with carbon, oxygen and hydrogen
- Inorganic phosphate ions are found in the cytoplasm of every cell - they have extra electrons (so have a negative charge and are therefore soluble in water)
- One of the fatty acid chains in a triglyceride molecule is replaced with a phosphate group to make a phospholipid
- Phospholipids have a charged hydrophilic head and a non-polar hydrophobic tail
- In water, they will form a layer on the surface with the phosphate heads in the water and fatty acids tails sticking out of the water (surface active agents - surfactants)
- Can also form a two layer sheet (phospholipid bilayer) where the tails face inwards and the heads face out. The tails are protected from the water by the hydrophilic heads.
- Play a key role in forming cell membranes
- They're able to seperate aqueous environment from aqueous cytosol within cells

Sterols
- Also known as steroid alcohols
- Another type of lipid found in cells
- They're not fats or oils
- They're complex alcohol molecules based on a four carbon ring structure and a hydroxyl group at one end.
- Hydroxyl group is polar and therefore hydrophilic, and the rest of the molecule is hydrophobic
- Cholesterol is a sterol
- Cholesterol is primarily manufactured in the liver and intestines
- Cholesterol has a role in the formation of cell membranes - positioned between phospholipids with hydroxyl group at the periphery of the membrane
- Cholesterol adds stability to membranes, keeping them fluid at low temperatures and stopping them becoming too fluid at high temperatures
- Vitamin D, steroid hormones and bile are all manufactured using cholesterol
Roles of Lipids
Due to their non-polar nature, lipids have many biological roles:
- membrane formation and the creation of hydrophobic barriers
- hormone production
- electrical insulation necessary for impulse transmission
- waterproofing, for example in birds' feathers and on plant leaves
Lipids, triglycerides in particular, also have an important role in long-term energy storage. They are stored under the skin and around vital organs, where they also provide:
- thermal insulation to reduce heat loss for example, in penguins
- cushioning to protect vital organs such as the heart and kidneys
- buoyancy for aquatic animals like whales
Emulsion Test for Lipids
1) The sample is mixed with ethanol.
2) The resulting solution is mixed with water and shaken.
3) If a white emulsion forms as a layer on top of the solution, this indicates the presence of a lipid. If the solution remains clear, the test is negative.

Proteins Introduction
- All proteins contain the elements carbon, hydrogen, oxygen and nitrogen
- Made of polymers called polypeptide chains
- Polypeptide chains are made of amino acid molecules (the monomer)
- The bonds between amino acids are peptide bonds
- Proteins consist of one or more polypeptides that are arrange as complex macromolecules
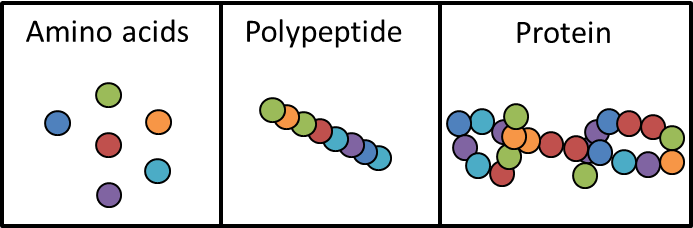
Amino Acids
- 20 different amino acids commonly found in cells
- 5 of these are non-essential as our bodies are able to make them from other amino acids
- 9 are essential and can only be obtained from what we eat
- 6 are conditionally essential as they are only needed by infants and growing children
Synthesis of Peptides
- amino acids join when the amine and carboxylic acid groups react
- R - groups not involved at this point
- hydroxyl from carboxylic acid group and hydrogen from amine group react
- a peptide bond forms and water is produced (e.g. of condensation reaction)
- resulting compound is a dipeptide
- many amino acids join to form polypeptide (reaction catalysed by peptidyl transferase)
- different R - groups interact with each other forming different bonds - these bonds lead to polpeptides folding into proteins
Primary Structure of Proteins
- sequence of amino acids
- directed by info carried within DNA
- the particular amino acids in the sequence will influence how the polypeptide folds to give the protein's final shape - this determines function
- only bonds involved in primary structure are peptide bonds

Secondary Structure of Proteins
- the oxygen, hydrogen and nitrogen atomes of the basic, repeating structure of the amino acids (variable groups not involved) interact
- hydrogen bonds may form within amino acid chain, pulling it into a coil shape called an alpha helix
- polypeptide chains can also lie parallel joined by hydrogen bonds, forming sheet-like structures
- pattern formed by individual amino acids causes structure to appear pleated - beta pleated sheet
- secondary structure is the result of hydrogen bonds and forms at regions along long protein olecules depending on the amino acid sequences
Tertiary Structure of Proteins
- folding of a protein into its final shape
- often includes sections of secondary structure
- coiling or folding of sections of proteins into their secondary structure brings R-groups of different amino acids closer together so they are close enough to interact and further folding of these sections will occur
- Between R-groups there are:
~ hydrophobic and hydrophilic interactions - weak interactions between polar and non-polar R-groups
~ hydrogen bonds - weakest of the bonds formed
~ ionic bonds - stronger than hydrogen bonds and form between oppositely charge R-groups
~ disulfide bonds - (aka disulfide bridges) covalent and strongest of bonds formed but only form between R-groups that contain sulfur atoms
Quaternary Structure of Proteins
- results from association of two or more individual proteins called subunits
- interactiosn between subunits are same as in the tertiary structure except they are between different protein molecules rather than within one molecule
- protein subunits can be identical or different
- enzymes often consist of two identical subunits
- insulin (a hormone) has two different subunits
- Haemoglobin has four subunits - made up of two sets of two identical subunits

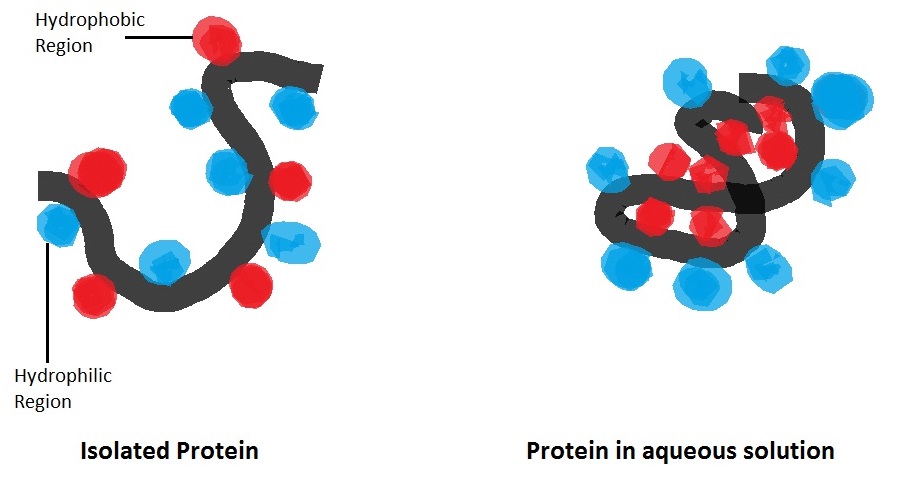
Hydrophilic & Hydrophobic Interactions
- proteins are assembled in aqueous environment of cytoplasm
- so the way in which a protein folds will also depend on whether the R-groups are hydrophilic or hydrophobic
- hydrophilic groups are on the outside of the protein
- hydrophobic groups are on the inside of the molecule shielded from water in the cytoplasm
Breakdown of Peptides
- proteases are enzymes that catalyse the reaction
- peptides are broken down into their constituent amino acids
- a water molecule is used to break the peptide bond in a hydrolysis reaction, reforming the amine and carboxylic acid groups
Biuret Test for Proteins
Peptide bonds form violet coloured complexes with copper ions in alkaline solutions.
1) A liquid sample is mixed with an equal volume of sodium hydroxide solution
2) copper sulfate solution is added a few drops at a time.
3) Positive result - purple colours solution forms
4) Negative result - solution remains blue

Globular Proteins
- compact, water soluble and usually roughly spherical in shape
- form when proteins fold into their tertiary structures in such a way that the hydrophobic R-groups are kept away from the aqueous environment
- hydrophilic R-groups are on the outside of the protein - so they are soluble in water
- this solubility is important as: they are essential for regulating many of the processes necessary to life (e.g. chemical reactions, immunity, muscle contraction)
- Insulin is a globular protein.
- hormone involved in the regulation of blood glucose concentration
- hormones are transported in the bloodstream so need to be soluble
- hormones also need to fit into specific receptors on cell-surface membranes to have their effect and therefore need to have precise shapes
Conjugated Proteins
- They're globular proteins that contain a non-protein component called a prostheic group
- lipipds or carbohydrates can combine with proteins forming lipoproteins or glycoproteins
- metal ions and molecules derived from vitamins also form prosthetic groups - cofactors when they are necessary for the proteins to carry out their functions
- Haem goups are examples of prosthetic groups; they contain an iron(II) ion (Fe2+)
- catalase and haemoglobin both contain haem groups
- Haemoglobin is the red, oxygen-carrying pigment found in red blood cells. It is a quaternary protein made from 4 polypeptides (2 alpha and 2 beta subunits). Each subunit contains a prosthetic haem group. The Fe2+ ions are able to combine reversibly with an O2 molecule. This is what enables haemoglobin to transport oxygen around the body.
- Catalase is an enzyme (enzymes catalyse reactions, so increase reaction rates and each enzyme is specific to a particular reaction or type of reaction). Catalase is a quaternary protein containing 4 haem prosthetic groups. The Fe2+ ions allow catalase to interact with hydrogen peroxide and speed up its breakdown. Hydrogen peroxide is a common byproduct or metabolism but is damaging to cells and cell components if allowed to accumulate. Catalase makes sure this does not happen.
Fibrous Proteins
- formed from long insoluble molecules
- due to the presence of a high proportion of amino acids with hydrophobic R-groups in their primary structures
- contain a limited number of amino acids, usually with small R-groups
- the amino acid sequence in the primary structure is usually quite repetitive - leads to very organised structres reflected in the roles fibrous proteins have
- Keratin, elastin and collagen are examples of fibrous proteins
- Keratin - group or fibrous proteins present in hair, skin and nails, has a large proportion of the sulfur-containing amino acid, cysteine, results in many disulfide bonds, forming strong, inflexible, and insoluble materials. Hair contains fewer bonds making it more flexible than nails, which contain more bonds.
- Elastin - fibrous protein found in elastic fibres, elastic fibres as present in walls of blood vessels and in alveoli. Gives them flexibility to expand when needed, but also to return to normal size. Elastin is a quaternary protein made from stretchy molecules called tropoelastin.
- Collagen - connective tissue found in skin, tedons, ligaments and nervous system. There are a number of different forms but all are made up of 3 polypeptides wound together in a long and strong rope- like structure. Has flexibility
Nucleic Acids and Nucleotides
- nucleic acids contain carbon, hydrogen, oxygen, nitrogen and phosphorus
- made from many nucleotides (monomer)
- nucleotides are made up of: a pentose monosaccharide, a phosphate group and a nitrogenous base
- nucleotides are linked together by condensation reactions to form a polymer called a polynucleotide
- phosphate group on one nucleotide forms a covalent bond with hydroxyl group on adjacent nucletoide - bond is called a phosphodiester bond
- this forms a long, strong sugar-phosphate backbone with a base attached to each sugar
- phosphodiester bonds are broken by hydrolysis
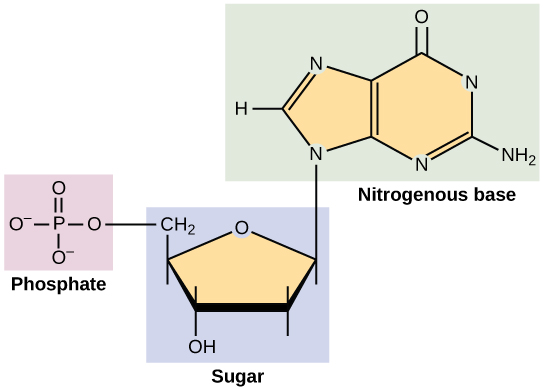
Deoxyribonucleic Acid (DNA) & Double Helix
- sugar - deoxyribose (a sugar with one fewer oxygen atoms than ribose)
- nucleotides in DNA each have one of four different bases - there are 4 different DNA nucleotides
- pyrimidines - smaller bases which contain single carbon ring structures (thymine and cytosine)
- purines are larger bases which contain double carbon ring structures (adenine and guanine)
- DNA is made up of two strands of polynucleotides coiled into a helix (DNA double helix)
- two stands of double helix are held together by hydrogen bonds between the bases
- each strand has a phosphate group at one end and a hydroxyl group at the other end
- two parallel strands are arranged so that they run in opposite directions - said to be antiparallel
- pairing between bases allows DNA to be copied and transcribed
Base Pairing Rules
- adenine and thymine form two hydrogen bonds and always join together
- cytosine and guanine form three hydrogen bonds and always join together
- called complementary base pairing
- these rules mean that a small pyrimidine base always binds to a larger purine base - arangement maintains constant distance between the DNA 'backbones' resulting in parallel polynucleotide chains
- DNA always has equal amounts of adenine and thymine and equal amounts of cytosine and guanine
- the sequence of bases along a DNA strand carries the genetic information of an organism in the form of a code
Ribonucleic Acid (RNA)
- plays essential role in transfer of genetic information from DNA to the proteins that make up the enzymes and tissues of the body
- DNA is a very long molecule and is unable to leave the nucleus in order to supply the info directly to the sites of protein synhesis
- to solve this, the relatively short section of the long DNA molecule corresponding to a single gene is transcribed into a similarly short messenger RNA (mRNA) molecule
- sugar is ribose rather than deoxyribose
- thymine base is replaced with the base uracil - still forms two hydrogen bonds with adenine
- after protein synthesis, the RNA molecules are degraded in the cytoplasm - phosphodiester bonds are hydrolysed and the RNA nucleotides are released and reused
DNA Extraction
- Grind sample in a mortar and pestle - breaks down cell walls
- Mix sample with detergent - breaks down cell membrane releasing the contents of the cell
- Add salt - breaks down hydrogen bonds between DNA and water molecules
- Add protease enzyme - breaks down the proteins associated with the DNA in the nuclei
- Add a layer of alcohol (ethanol) on top of the sample - causes the DNA to precipitate out of solution
- DNA will be seen as white strands forming between the layer of sample and layer of alcohol - DNA can be picked up by 'spooling' it onto a glass rod
DNA Replication & Genetic Code
- Semi-conservative replication - the two new molecules of DNA produced consist of one old strand and one new strand of DNA
- Before replication can occur, unwinding and separating of the two strands of the DNA double helix is carried out by the enzyme DNA helicase
- Free nucleotides pair with the newly exposed bases on the template strands
- DNA polymerase (another enzyme) catalyses the formation of phosphodiester bonds between the nucleotides
- sequences of bases are not always matched exactly and an incorrect sequence may occur in the newly-copied strand - these errors occur randomly and spontaneously and lead to a change in the sequcne of bases (mutation)
- DNA must code for a sequence of amino acids - genetic code
- code in the base sequences is a simple triplet code - sequence of three bases - codon
- each codon codes for an amino acid
- a section of DNA that contains complete sequence of bases to code for an entire protein is called a gene
- genetic code is non overlapping
- many amino acids can be coded for by more than one codon - code is degenerate
- 64 different codons but only 20 amino acids
Transcription
- only one of the two strands of DNA contains the code for the protein to be synthesised - sense strand (runs from 5' to 3')
- other strand is a complementary copy of the sense strand and does not code for a protein - antisense strand (runs from 3' to 5') this acts as the template strand
- free RNA nucleotides will base pair ith complementary bases exposed on the antisense strand
- phosphodiester bonds are formed between the RNA nucleotides by the enzyme RNA polymerase
- transcription stops at end of gene and completed short strand of RNA is called messenger mRNA
- mRNA detaches from DNA template and leaves nucleus through nuclear pore
- DNA double helix reforms
- mRNA molecule travels to a ribosome in the cell cytoplasm
Translation
- in eukaryotic cells, ribosomes are made up of two subunits, one large and one small
- subunits are composed of almost equal amounts of protein and a form of RNA called ribosomal rRNA - important in maintaining structural stability of protein synthesis sequence and plays a biochemical role in catalysing the reaction
- mRNA binds to small subunit of ribosome at its start codon
- tRNA with complementary anticodon binds to the mRNA start codon - tRNA carries the amino acid
- second tRNA binds to the next codon - second amino acid also arrives
- a peptide bonds form between the two amino acids - catalysed by peptidyl transferase - an rRNA component of ribosome
- ribosome then moves along mRNA, releasing first tRNA
- process repeated until ribosome reached end of mRNA at a stop codon and polypeptide is released
- many ribosomes can follow on the mRNA behind the first so that multiple identical polypeptides can be synthesised simultaneoulsy
ATP
- cells require energy for three main types of activity - synthesis, transport, movement
- inside cells, molecules of adenosine triphosphate (ATP) are able to supply this energy in such a way that it can be used
- ATP molecule is composed of a nitrogenous base, a pentose sugar and 3 phosphate groups - it is a nucleotide
- however, in ATP the base is always adenine and there are 3 phosphate groups instead of one
- sugar in ATP is ribose
- ATP is used for energy transfer in all cells of all living things, hence it is known as the universal energy currency
How ATP Release Energy
- small amount of energy needed to break the relatively weak bond holding the last phosphate group in ATp
- large amount of energy is release when the liberated phosphate undergoes other reactions involving bond formation
- overall, more energy is released than used
- water is involved in the removal of the phosphate group - hydrolysis reaction
- hydrolysis of ATP does not happen in isolation but is associated with energy-requiring reactions - reactions are said to be couples as they happen simultaneously
- ATP is hydrolysed into adenosine diphosphate (ADP) and a phosphate ion, releasing energy
- ATP not a good long-term energy store due to instability of phosphate bonds (fats and carbs much butter)
- create ATP by reattaching a phosphate group to an ADP molecule - phosphorylation (as water is removed it is another example of a condensation reaction)
- due to instability of ATP cells do not store large amounts of it
- interconversion of ATP and ADP is happening constantly in all living cells, meaning cells do not need a large store of ATP
- ATP is therefore a good immediate energy store
Properties of ATP
- small - moves easily into, out of and within cells
- water soluble - energy-requiring processes happen in aqueous environments
- contains bonds between phosphates with intermediate energy - large enough to be useful for cellular reactions but not so large that energy is wasted as heat
- releases energy in small quantities - quantities are suitable to most cellular needs, so that energy is not waster as heat
- easily regenerated - can be recharged with energy
Related discussions on The Student Room
- Any good youtube channels for Bio + Chem a levels? »
- Whats beter revising a whole topic for 1 subject or 2 lessons for 2 subjects A level »
- Paper 3 AQA a Level biology »
- AQA A Level Biology Practice Questions (chapter 1) »
- Do I need to know how to draw structures for carbohydrates? (AQA A Level Bio) »
- AQA A Level Biology »
- 25 mark essay question »
- A-level Biology Study Group 2023-2024 »
- BTEC applied science Unit 10 »
- Biology AS Question Help »

Comments
No comments have yet been made