C3- Structure and bonding
- Created by: adrenovci
- Created on: 30-05-18 12:05
C3.1- States of matter
The particle theory
Solids: fixed/ definite shape, strong forces of attraction between particles, don't move from their positions (only vibrate)
Liquids: move past eachother, weak force of attraction, constantly moving in random motion
Gases: far apart, VERY weak force of attraction, constantly moving in random motion, travel in straight lines
- A solid turns to liquid at melting point. The hotter it is, the faster the particles vibrate. The vibrations will be so strong the particles break free of eachother, melting into liquid.
- Liquid turns into gas a boiling point. As the temperature rises, the particles try to to escape the surface of the liquid. Its rate of evapouration increases, eventually buuble of gas rise and escape the liquid.
- Each change of state is reversible. In melting and boiling, energy is transferred from the surroundings to the substance. In freezing and condensing, energy is transferred from the substance to the surroundings.
C3.2/ C3.3- Ionic bonding
Ionic bonding- The transferring of electrons to achieve a stable electronic structure
When a metal and non-metal react together, the metal atom loses electrons to form a positively charged ion and the non-metal electron gains these electrons to form a negatively charged ion. These oppositely charged ions are strongly attracted to one another by electrostatic forces. The attraction is an ionic bond. They result in an arrangement called a giannt structure/ lattice.
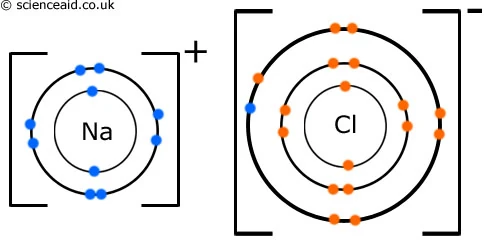
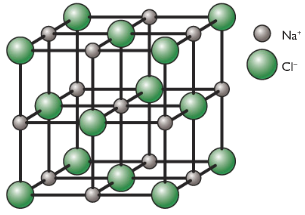
C3.4- Giant ionic structure
Ionic compounds have a structure called giant ionic lattice. The ions form a closely packed regualr lattice arrangement and there are very strong electrostatic forces of attraction between oppositely charged ions, in all directions in the lattice.
- Have high melting and boiling points due to the many strong bonds between the ions. It takes a lot of energy to overcome this attraction.
- When they're solid, the ions are held in place, so the compounds cant conduct electricity.
- When they melt, the ions are free to move within the molten compund and they'll carry electric current and can conduct electricity.
- Some ionic compounds dissolve in water. The ions seperate and are all free to move, so they too carry electrical current and can conduct electricity.
C3.5- Covalent bonding
Covalent bonding is when two non-metal atoms share electrons to achieve a stable electronic structure. The positively charged nuclei of the bonded atoms are attracted to the shared pair of electrons by electrostatic forces, making covalent bonds very strong. They take a lot of energy to break apart. Atoms only share electrons on their outer shells.
C3.6- Structure of simple molecules
Each molecule tends to be quite seperate form its neighbouring molecules. The force of attraction between the individual molecules in a covalent substance is relatively small- there are weak intermolecular forces between molecules. Overcoming these forces don't take much energy.
Intermolecular forces increase with the size of the molecules, so larger molecules have higher melting and boiling points. Compounds made of simple molecules do not conduct electricity, even when molten or dissolved in water. This is because there is no overall charge, so their neutral molecules cannot carry electrical charge.
Polymers are made up of many small reactive molecules that bond to eachg other to form long chains. E.g. poly(ethene) is made up of thousnads of small ethene molcules,(C2H4)n ,reacting together. Because polymers are long chains, the intermolecular forces are larger than between simple covalent molecules, so more energy is needed to break them. This makes polymer solid at room temperature.

C3.7- Giant covalent structures
In giant covalent structures, all the atoms are bonded to each other by strong covalent bonds. Giant covalent structures:
- Have very high melting points and boiling points, as a lot of energy is need to break the covalent bonds
- They are insoluble in water
- Apart from graphite, they don't contain charged particles, so they don't conduct electricity. They are also hard.
E.g. Diamond, graphite, and silicon dioxide (silica)
In graphite, each carbon atom only forms three covalent bonds, creating sheets of carbon atoms arranged in hexagons. There aren't any covalent bonds between the layers, only weak intermolecular forces holding them together, so they're free to move over each other. This makes graphite soft and slippery, so its ideal as a lubricating material. Only three out of each carbon's four outer electrons are used in bonds, so each carbon atom has one delocalised (free) electron. This allows the graphite to conduct electricity and thermal energy, unlike diamond which has no free electrons.
C3.8- Fullerenes and graphenes
Fullerenes are molecules of carbon, shaped like closed tubes or hollow balls. They're mainly mad up of carbon atoms arranged in hexagons, but can also contain pentagons or heptagons. Fullerenes can be used to "cage" other molecules. The fullerene structure forms around another atom or molecule, which is then trapped inside. This could be used to deliver a drug into the body.
Fullerenes can form nanotubes - tiny carbon cylinders. The ratio between the length and the diameter of nanotubes is very high. They have useful properties, such as:
- high tensile strength (they don't break when stretched)
- high electrical conductivity and high thermal conductivity due to their delocalised electrons.
Graphene is a sheet of carbon atoms joined together in hexagons. The sheet is just one atom thick, making it a 2D compound. Like graphite, it contains delocalised electrons so can conduct electricity and thermal energy. It also has a very low density, is the most reactive form of carbon, and pieces of it are incredibly strong for their mass.
C3.9/ C3.10- Metallic bonding/ Alloys
Metallic bonding
Metals also consist of giant structures The electrons on the outer shell of the metal atoms are delocalised. There are strong forces of electrostatic attraction between the posistive metal ions and the shared negative electrons. These forces of attraction hold the atoms together in a regular structure and are known as metallic bonding. Metallic bonding is very strong. Substances that are held together by metallic bonding include metallic elements and alloys. Its the delocalised electrons in the metallic bonding which produce all the properties of metals.
Alloys
Pure metals are too soft so are mixed with other metals to make them harder. An alloy is a mixture of two or more elements, at least one of which is a metal. Different elements have different sized atoms. So when another element is mixed with a pure metal, the new metal atoms will distort the layers of metal atoms, making it more difficult for them to slide over each other. This makes alloys harder than pure metals.
C3.11- Nanoparticles
Nanoscience is the science of very small things. It deals with structures that are just a few hundred atoms in size or even smaller.
The surface area to volume ratio is an important factor as it affects the way that a particle behaves. Surface area to volume ratio = surface area / volume
As particles decrease in size, the size of their surface area increases in relation to their volume. This causes the surface area to volume ratio to increase.
Nanoparticles have a very high surface area to volume ratio - this means the surface area is very large compared to the volume. This can cause the properties of a material to be different depending on whether it's a nanoparticle or whether it's in bulk. E.g. you'll often need less of a material that's made up of nanoparticles to work as an effective catalyst compared to a material made up of "normal" sized particles (containing billions of atoms rather than a few hundreds).
C3.12- Apllications of nanopartocles
Here are some of the uses of nanoparticles:
- Nanomedicine - the idea is that tiny particles are absorbed more easily by the body than most particles. This means that they can deliver drugs right into the cells where they're needed.
- Some nanoparticles conduct electricty, so they can be used in tiny electric curcuits for computer chips.
- Silver nanoparticles have antibacterial properties. They can be added to polymer fibres that are then used to make surgical masks and wound dressings and they can also be added to deodorants.
- Nanoparticles are also being used in cosmetics. E.g. they're used to improve moisturisers without making them really oily.
However we don't fully understand the effects of nanoparticles on our bodies:
- Some people are worried that products containing nanoparticles have been made available before the effects on human health have been investigated properly.
- It is possible that nanoparticles might wash away and damage the environment
- Breathing in tiny particles could damage the lungs. Nanoparticles could enter the bloodstream this way, or from their use in cosmetics, with unpredictable effects on our cells.
Comments
No comments have yet been made