Biology AS1
- Created by: Mark Kelly1
- Created on: 22-05-17 19:24
The Role of Biologically Important Ions
Nitrate- component of Amino Acids, Nucleic Acids and Chlorophyll
Calcium- Calcium Pectate in Plants for middle Lamella, improtant component for bones and teeth and blood clotting and muscle contraction
Iron- part of Haem group in Haemoglobin and important consituent of electron carriers in respiration
Magnesium- chlorophyll
Potassium- maintaining electrical gradients across Neurons
Phosphate- phospholipids, phosphate is important for cell membranes- Major component in ATP and Nucleic Acids
Hydrogencarbonate- Natural Buffer
Types of Carbohydrates
Monosaccharides- basic carbohydrate monomer ie simple sugars- trioses pentoses or hexoses usually eg. Glucose and Fructose
Disaccharides- double sugars formed from two monosaccharide monomers eg. Sucrose and Maltose
Polysaccharides- complex molecules consisting of many monosaccharide monomers.
Monosaccarides
a-glucose and beta- glucose
a-glucose-hydroxyl group on bottom
beta-glucose -hydroxyl group on top
Monosaccharides have the same molecular formula but different structural formula therefore known as Structural Isomers
Disaccharides
Two monosaccharides usually Hexoses react together in a condensation reaction .This reaction is reversable and is known as Hydrolysis(adding water)
The bond formed between the two monosaccharides is known as a Glycosidic Bond

Example of Important Disaccharides
Maltose= a-glucose + a-glucose (Formed when starch is digested)
Sucrose = a- glucose + fructose
Disaccharides Characteristics
Disaccharides have the general formula C12H22O11
They dissolve in water producing sweet tasting solutions
Polysaccharides
Complex Carbohydrates with often very long chains
Formed through Condensation and broken by Hydrolysis
Large number of monomers joined together to make a complex Polymer
Not sugar! They are not sweet and are insoluble in water
eg. Starch, Glycogen and Cellulose
Starch Structure
Amylose= a-glucose linked by a-1,4 glycosidic bond. Coils forming a spiral held together by hydrogen bonds. Due to only a-1,4 bonds Amylose forms a long unbranched chain. Due to the presence of bulky sidegroups that cause the a-glucose molecules to lie at different angles , amylose chains form a coiled configuration
Amylopectin = a-glucose linked by a-1,4 glycosidic bonds and a-1,6 glycosidic bonds. The a-1,6 glycosidic bonds form side brances to produce a branched molecule. This branching occure as often as 1 in every 10 a-glucose monomers
Why is Starch good for Storage?
Amylose and Amylopectin are both very compact(aided by the coiled configuration). Therefore they contain a rich store of glucose in a small space.
They are insoluble thererefore will not affect the water relations of the cell (Osmotically Inert)
Starch is a large molecule so it can be retained within the cell and will not pass through the cell surface membrade readily
The brancing nature of amylopectin means that there is many terminal ends which can be easily hydrolysed. This aids rapid enzymatic breakdown of starch into its consituent glucose molecules ar times of high respiratory demand
Glycogen
Storage Carbohydrate in Animal and Fungal Cells - Stored as small granules
a-1,4 glycosidic bonds and a-1,6 glucosidic bonds
Glycogen is more branched and shorter than Amylopectin
Stored in Liver and Muscle Cells
Compact and insoluble
No unbranched chains so more terminal ends so faster hydrolysis when conditions demand
Cellulose
Not a storage polysaccharide- its role is purely structural
Made from beta-glucose monomers joined together by B-1,4 glycosidic bonds - must rotate 180 due to the different structure (hydroxyl group on top)
This flipping has 2 effects:
- Straigher chains as bulky CH2OH groups alternate
- Hydrogen Bonds can form cross linkages between adjacent chains
Cellulose chains grouped together in microfibrils with each microfibril consisting of many cellulose molecules. Plant cell walls are comprised of many cellulose microfibrils orientated into many planes in a lattice structure to further increase the tensile strength.
Lipids
Hydrogen to Oxygen 2:1
Large Molecules
Hydrophobic- not soluble in water
They are soluble in organic solvents such as ether and ethanol
Main Types are Tryglicerides( Oils and Fats) , Phospholipids ( major component of membranes),Waxes and Steroids
Triglycerides
Combination of Glycerol and 3 Fatty Acids( Hydrocarbon Chains).
Fatty Acids are organic acids that form long hydrocarbon tailes linked to a Carboxyl Group (COOH)
Ester Bond forms between Glycerol and Hydrocarbon Tail

Saturation and Unsaturation
Saturated Fatty Acids contain the maximum number of hydrogen atoms . Linked by a C-C single bond. eg.Stearic Acid
Unsaturaturated Fatty Acids contain atleast one C=C double bonds in the chain.eg. Oleic Acid
If there is one C=C double bond it is monounsaturated.
If there is more than one C=C double bond it is Polyunsaturated
Tryglcerides as Energy Stores
Tryglycerides are an excellent energy store as they release more energy per unit per mass than carbohydrates.
Fats are also important for insulation and are stored in a layer below the body surface in many animals. Many body organs are protected by a layer of fat. Fat is important for boyancey in marine life etc
Phospholipids
One Glycerol, 2 Fatty Acids and a Phosphate.
The fatty acid molecules repel water and are insoluble in water forming hydrophobic tails whereas phosphate gives the glycerol parts of the molecule are hydrophillic properties and is soluble in Water. Phospholipids are polar molecules and this property is important in determining their orientation and function in the cell surface membrane
Proteins
Contains Carbon, Oxygen, Hydrogen,Nitrogen and Usually Sulfur
They are large Polymers formed form amino acid sub-units.
Proteins are determined by the sequence of the amino acids in the protein.
The Amino Acids differ due to them having different R-groups

Dipeptide
Amino Acids are linked together by peptide bonds . These condensation reactions with hte loss of water. The Condensation reaction takes place between the amino group of one of the amino acids and the carboxyl group of another.This link is between the Nitrogen and Carbon Atoms involved.Thebond formed is a Dipeptide.
Primary Protein Structure
The sequence of amino acids in the polypeptide chains. Its when the amino acids are linked by the peptide bonds
Secondary Protein Structure
The Orientation of how the Hydrogen bonds are orientated.
a-helix- Hydrogen bonds formed at regular intervals between amino acids. The bonds twist the chain of amino acids into a spiral or helical shape
B-pleated sheets- more rigid and less flexible configurations that the a-helix,
They are sections formed by the polypeptide chain, orientated in oppiside (anti-parrallel) directions, laying adjacent to eachother. Hydrogen bonds form between the C=O and NH groups
Tertiary Protein Structure
Further folding of the secondary structure. Unique 3-D Shape and is a consequence of the range of bonds formed between the R-Groups of the Amino acids in the chain,
Hydrogen Bonds-numerous and relatively weak and easily broken
Ionic Bonds- between amino and carboxyl groups. Stronger than hydrogen bonds but damaged by changes in pH
Disulfide Bonds- covalent bonds formed between R-groups of sulfur containing Amino Acids. Very Strong bonds and important in giving structual strength to fibrous proteins eg Collagen
Hydrophobic Interactions- Amino Acids with hydrophobic R-groups whoch tend to take up positions within the molecule surrounded by other parts of the polypeptide further influencing the tertiary structure.
Quaternary Protein Structure
Two or more polypeptides bonded together.
Some Quaternary Structures contain prostethic groups that are integral to their function.
These conjugated proteins include glycoproteins important for membrane structure
Haemoglobin is a conjugated protein that consists of 4 polypeptide chains and 4 Iron Rich Haem groups which is essential for the transport of 02 in the blood.
Fibrous Proteins- polypeptides arranged in chains that form fibres or sheets. The parallel chains are linked by cross bridges which form very stong and stable molecules. Fibrous Protiens are invariably structural in function. eg Collagen- Found in tendons which link muscle to bone
Globular Proteins- have metabolic role and include Enzymes and Antibodies. The ability of the protein to form a very specific 3D shape is crucial to their role are enzymes and antibodies eg. Haemoglobin
Prions
Particular type of Protein found in Mammals and in some other animal groups. Found in the Nervous System and is thought to be involved in synaptic transmissionThe Normal Prion Protein can convert to the disease casuing Prion (PrPc to PrPSc)Normal PrPc consists of more a-helices however PrPSc constins more B-pleated Sheets.Leads to a chain reaction - leads to Neurodegenerative disorders in the brain and other nervous tissues and eventually death
Prions can arise
- Through Consumption of contaminated Protein
- Sproadically/Spontaneously
- Mutations in DNA that codes for the normal PrPc Protein
Forms of Prions Disease
- Scrapie- Sheep
- BSE-Cows
- vCJD- Humans
Nucleic Acid
Consists of
- Pentose Sugar
- Phosphate Group
- Nitrogenous Base
The three components are joined by condensation reactions./ broken down by Hydrolysis
Phosphodiester bond links pentose sugar to the phosphate (Sugar-Phosphate Backbone)
Adjacent Nucleotides can be joined together to create the Nucleic Acid. The Nucleic Acid is a chain of Nucleotides.(Polynucleotide)

Nucleic Acid
Consists of
- Pentose Sugar
- Phosphate Group
- Nitrogenous Base
The three components are joined by condensation reactions./ broken down by Hydrolysis
Phosphodiester bond links pentose sugar to the phosphate (Sugar-Phosphate Backbone)
Adjacent Nucleotides can be joined together to create the Nucleic Acid. The Nucleic Acid is a chain of Nucleotides.(Polynucleotide)

DNA and RNA
RNA
Three Types
- messanger RNA(mRNA)
Carries code from the DNA in the nuceus to a ribosome in the cytoplasm where protein systhesis occurs
- transfer RNA(tRNA)
Carries the Amino Acids to the mRNA/Ribosome where the protein synthesis is taking place. It is a single chain folded into a clover leaf shape.
- ribosomal (rRNA)
Made in the Nucleolus and forms over half the mass of each individual ribosome
DNA Replication
Semi Conservative Mechanism- genetic replication in which a double-stranded molecule of nucleic acid separates into two single strands each of which serves as a template for the formation of a complementary strand that together with the template forms a complete molecule.
1. The enzyme DNA Helicase breakes the Hydrogen Bonds between the base pairs and 'unzips' part of the DNA double helix revealing two strands
2. The DNA Polymerase moves along each strand which acts as a template fot the synthesis of a new strand
3. DNA Polymerase catalyses the joining of free deoxyribonucleotides to each of the exposed original strands according to base pairing rules that the new complimentary strands form.
4. The process of unzipping and joining for new nucleotides continues alond the whole length of the DNA molecule
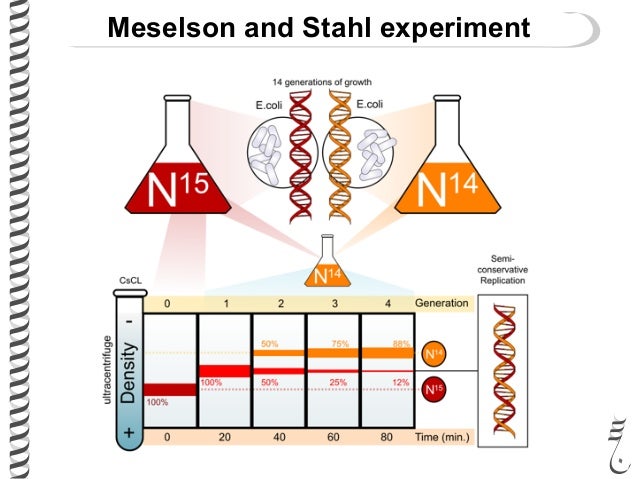
Meselson and Stahl Experiment
Meselson and Stahl tested the hypothesis of DNA replication. They cultured bacteria in a 15N medium. 15N is a heavy isotope of nitrogen so the DNA synthesized is of heavy density. They then shifted the bacteria to a 14N medium, After one generation, the DNA was all of intermediate density.
After two generations , two bands of DNA were seen, one of intermediate density and one of light density. This result is exactly what the semiconservative model predicts: half should be 15N-14N intermediate density DNA and half should be 14N-14N light density DNA. This result rules out the dispersive replication model, which predicts that after replication cycle 1, the DNA density of all DNA molecules will gradually become lower, so no intermediate density DNA should remain at 2nd Generation. The semiconservative model is correct.
Enzymes- Structure and Function
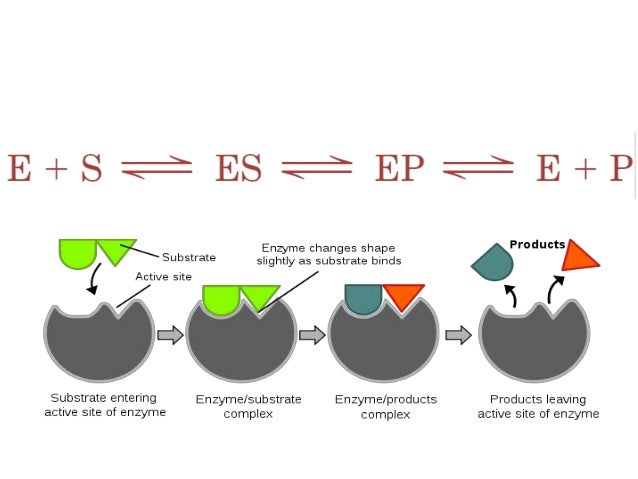
Enzymes are Biological Catalysts which speed up the rate of Metabolic Reactions.
Enzymes are globular proteins which is advantageous

Activation Energy changes
Enzymes lower the activation energy to overcome the energy barrier.
This reduction in activation energy enables reactions to take place at a more rapid rate needed to sustain life.
Enzyme Action
Anabolism= building up of material
Metabolism= breaking down of material
Enzymes have specific active sites ( Enzyme Specificity)
Lock and Key Hypothesis
Substrate and Enzyme Active Site complimentary.- Proves enzyme specificity
Induced Fit Model
The active site moulds itself around the substrate forming a precise fit. The active site is therefore flexible and as it changes shape to fit the substrate the enzyme is able to put pressure on the substrate breaking particular bonds and therefore lowering the activation energy required for the reaction to take place- When products released the activesite returns to pre-reaction shape.
Co-Factors
Non-protein substances that enzymes require in order to function.
Examples include Metal Ions (such as Mg2+, Ca2+ and Fe3+ ). They form attachments to the enzyme and change the shape of the active site, enabling the reaction to take place.
Co-Enzymes
Particuar Type of co-factor. They are non-protein ,organic molecules necessary ot the enzymes active. Unlike some other cofactors theyre are not permanently attached . Co-enzymes are very important in the biochemistry of respiration and photosynthesis. The coenzymes NAD and FAD act as the hydrogen acceptors in Respiration
Substrate Concentration
The Rate of reaction increases as substrate concentration increases. Substrate molecules all occupy activesites and substrate availability limits the rate of the reaction with some of the active sites unused.
The addition of extra substrate molecules has no effect as all the activesites are in use. The Number of enzyme active sites limits the rate of the reaction
Enzyme Concentration
As more enzymes and active sites become available more enzyme-substrate compleces can form and reactions can take place.
Enzyme Activity levels off as the number of substrate molecules becomming limiting.
Normally in living systems activity continues to increase as substrate is sledom limiting
Temperature
Increasing Temperature, increases the KE, molecules vibrate more vigourously and this leads to more bonds to break which effects the tertiary structure of the globular protein to be affected. This causes the Active site to change shape to cause little/no enzyme substrate complexes to form.
pH
Each enzyme has an optimum pH range in which the enzyme works best at . At either side of this optimum changes in pH will reduce the activity. This is caused by the changes in pH disrupting the bonds that are improtant in determining the protein shapr. The Ionic Bonds in particular are subject to disruption when in non opetimal pH. The further the pH is from the optimum the greater the degree of disruption to the bonding until eventuallt denaturing results.
Competitive Inhibition
Inhibitor Substances competes with the usual substrate for the active site. Competitive inhibitors are very similar in shape to the usual substrate
If the quantity of the inhibitor substance is low compared to the substrate it will have little or no effect. If it is relatively high it can significantly affect the enzyme activity
Non-Competitive Inhibititon
The inhibitor attaches itself to an allosteric site-other than the active site
The presence of the non competitive inhibitor leads to the active site changing shape so that it is no longer complementary to the substrate molecule.
Increasing the substrate concentration does not reduce the effect of the inhibitor as the two molecules are not in direct comeptition

Elastase as Biomarker
Elastase is an enzyme released by white blood cells as a consequence of lung infection. - breaks down the bacterial pathogens and is apart of the immune response
Elastase breakes down the protein Elastin which is an important component of lung tissue that contributes to the elasticity of the alveoli.The elastin allows the alveoli to stretch and recoil. Normally when its not broken downs the elastase inhibitor alpha-1-antitrypsin prevents the Elastase activity after the infection has been cleared
However cigarette smoke causes too much Elastase to be released in the lungs with little A1AT present to control the elastase effectively . Therefore the elastin in the Alveoli is broken down resulting in Elastase-Induced Emphysema
Enzyme Inhibitors as Therapuetic Drugs
A1AT- used medically to reduce the harmful effects arising from Lung Infection
ACE(Angiotensin Convering Enzyme)- used to treat HBP and other Cardiovasular disases by inhibiting an enzyme that leads to the constriction of the blood vessels . The ACE inhibitors reduce the constriction of blood vessels - lowering BP
Penicillin- inhibiting enzymes involved in cell wall formation in bacteria. Some antiviral drugs inhibit DNA/RNA Polymerase preventing viral replication
Enzyme Immobilisation
Adsorption- Attached by weak forces onto an inert substance such as glass or a matrix.
Entrapment- trapped within polymers such as alginate beads or microspheres
Encapsulation- trapped inside a selectively permeable membrane such as Nylon
Cross-Linkage- covalenly bonded to a matrix such as Cellulose as consequnce of chemical reactions

Enzyme Immobilisation Advantages
Increased Themal Stability /pH Stability
More resistant to changes in pH
Can be Retained and Reused
Commercial processes continuous- faster and less waste
No contamination of end product- simplifying downstreaming process reducing purification costs and avoiding any allergic reactions to consumers.
Enzyme Immobilisation Disadvantages
Reduced speed of diffusion
Byproduct
Enzyme may be wasted
Active site might be in wrong orientation
Active site may change shape
Research costs high
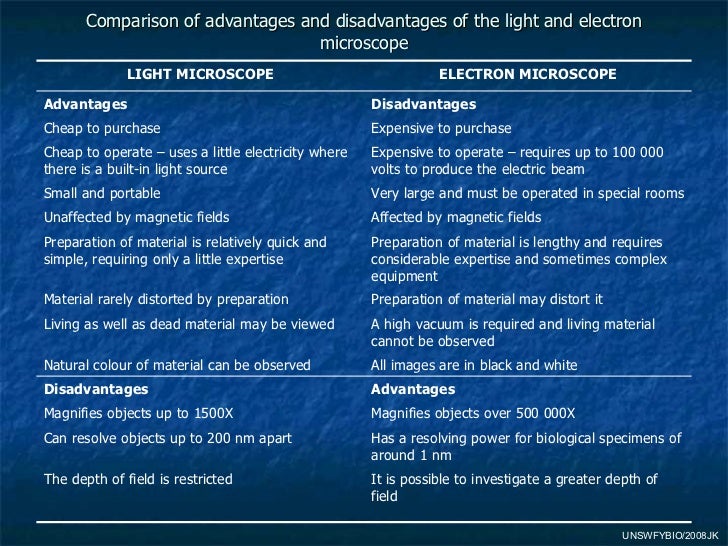
Light Microscope vs TEM
Eukaryotic Cell
Cell with Nucleus
Consist of cell surface membrane and cytoplasm and Nucleus with organelles
EUKARYOTIC CELLS FOUND IN ANIMALS PLANTS AND FUNGI
Animal Cells
Lysosomal Activity
Mitocondria

Nucleus

Plant Cells
Plant Cell Layers

Chloroplast structure
Lipid droplets also present
Fungal Cells
Contain
Chitin Cell Wall
SER + RER
Nuclei can be multinulceate
Vacuole
Glycogen Granules
Hyphae- long thread like structures-englongated cells
Cell Surface Membrane

Function of the Phospholipid Bilayer
The backbone of the cell surface membrane
Gives the membrance its selectivity or differentially permaeble properties

Cholesterol
Increases Membrane Stability by restricting the sideways movement of the phospholipid molecules at high temerature.
At low temperatures the cholesterol helps to maintain the membrane fluidity by acting as a wedge between adjacent phospholipid molecules and stopping adjacent molecules sticking together.
In effect Cholesterol helps make the membrane less fluid at high temperatures and more fluid at low temperatures
Proteins in Membrane
- Help provide stability and support as they anchor the phospholipid moleules.
- They may also act as Enzymes.
- Some proteins act as adhesion sites - areas where adjacent cells are held together
- Also some proteins are involved in cell recognition and as receptors or antigens
Important in transporting substances across through
- Channel proteins - span the membrane and work by creating a hydrophillic channel that allows the polar molecules to bypass the hydrphobic centre of the bilayer -gated/non-gated
- Carrier Proteins - carry specific ions and molecules across the membrane. May be because the moleucles/ ions have been moved against their concentration gradient. The Carrier Protiens can change shape to carry the substance from one side to another
Glycocalyx
Polysaccarides bind to the glycoprotein or glycolipid are found on the outise of the cell surface membrane and are peripheral to the protein or phospholipid.
Glycoproteins and Glycolipids involved in
- Cell-to-Cell recognition
- Antigens (glycoproteins)
- Receptor sites- important for hormone action and in the passage of Neurotransmitters between Neurons
- Glycoproteins and Glycolipids can form Hydrogen Bonds with water molecules outside the membrane so can increase stability of the membrane
Bacteriophages
Virus- commonly called phages
Normally have DNA core and are Parasitic on Bacteria
Inside their host cell the viral DNA codes for the production of new protein for new protein coats. The DNA itself replicates to make copeis that are packages within the protein coats forming new viruses .
Eventually due to the build up of viruses in the coat it ruptures releasing the viruses to continue the cell cycle

Human Immunodeficiency Virus
RNA Core.
Protein coat with a lipid bilayer containing glycoproteins.
Reverse Transcriptase enzyme catalyses the synthesis of DNA from RNA . DNA them makes new viruses by synthesising new protein coats and viral RNA
These viruses are known as Retroviruses as the viral RNA is used as a template to make DNA . This is the revers of the normal transcription process where DNA is used to make mRNA as part of the protien synthesis.
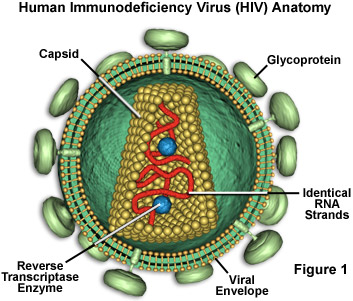
AIDS
In Humans HIV invades the lymphocyte - helper-T-cells.
These T cells are very important for the Immune System when protecting against disease.
As progressively more T-Cells are destroyed the immune system becomes critically comprimised and medical condition AIDS can develop
Diffusion
The net movement of a substance from where it is in highter concentration ot where it is in a lower concentration . Its not restricted to occuring across membranes
Factors Affecting Diffusion-
- Concentration Gradient - greater the conc. gradient faster diffusion
- Size of molecule- smaller molecule faster diffusion
- Temperature- high temp. inc diffusion
- Thickness of Exchange Surface- thin surface faster diffusion
- Surface Area of Membrane - greater SA faster diffusion
Facilitated Diffusion
Channel Proteins- Static- gated/non-gated. Allow charged particals to pass through.
Carrier Proteins- Take in the diffusing moleule such as glucose change shape and release the molecule on the other side of the membrane. These protein carriers have binding sites that match specific moleucles and they assist the movement of these molecules across the membrane
Active Transport
Moving molecules against their concentration gradient . Energy required- ATP
Active Transport involves protein carrier molecules(pumps). The substance binds to the carrier protein.
Cells that carryout Active Transport have high number of Mitocondria- important for synthesis of ATP energy

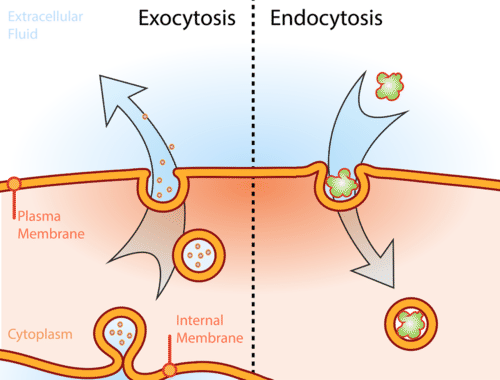
Cytosis
Endocytosis- movement of substances into the cell- cell wall invaginates and pinches off as vesicle
Exocytosis- movement of substances out of the cell-vesicle fuses with cell membrane
Phagocytosis- transporting solid material into cell
Pinocytosis- transport of fluid into cell
Osmosis
High water potential to lower water potential
Water potential is the tendancy to take in water by osmosis from pure water across a selectively permaeble membrane. Pure water was solute potential of 0kPa, Solutes decrease solute potential

Solute Potential is the potential of a solution to take in water Pressure Potential is the effect of pressure on the solution ( ONLY IN PLANT CELLS)
Water Potential Graphs
As cell begins to become more turgid the membrane begins to exert a force on the cell wall( Incipient Plasmolysis)At full Turgor the maximum force betweeen the cell and the cell walWater Potential of the cell is zero so no more water can enter Solute potential is still negative at full turgor as solutes are present in the cell As the pressure potential becomes positive it begins to hinder the water entering the cell therefore the cell potential and solute potential diverge As the cell takes in water by osmosis its contents become less concentrated therefore the solute potential increcreases. There is no pressure potential to restrict the water intakes therefore the solute potential=cell potential
Osmosis in Plant Cells

Osmosis in Animal Cells
Cell Cycle
Checkpoints

G0 Phase

Cell Cycle Regulation-Cancer
Cancer is casued by uncontrolled cell division in effect it is Mitosis out of control
Cancer cells cannot enter the G0 phase and cancer involves a breakdown in the ability of the checkpoints in the cycle to regulate the process of cell division.
Modern Anti-canver drugs- Chemotherapy
Inhibition of Microtubule formation= Vincristine binds to the tubulin in the microtubules ( spindle fibres) and prevents them from functioning properly . This stops the spindle fibres contracting and pulling the chromatids apart ( prevents Anaphase) VERCRISTINE IS A MITOTIC POISION
Antimetabolies act as S-Phase Inhibitors preventing DNA Synthesis.= Fluoroacil - inhibits an enzyme involved in making nucleotides that contain the base Thyamine. This prevents DNA synthesis. Chemotherapy Drugs can affect the normal cells as well ascancer cells . Anti Cancer drugs can also stop ordinary cells dividing. Leads to unpleasant side effects to the patients recieving Chemotherapy
Graph for Amount of DNA in Cell Cycle
DNA doubles during DNA replication and chromatid formation.
DNA is then Halved during Cytokinesase as DNA is divided between two daughter cells
Chromosomes
Chromasomes consist of an ectended DNA molecule sipported by special proteins called Histones. Chromatin condenses to form Visible Chromosomes during Nuclear Division
DNA coils tightly around the stack to form a structure called a Nucleosome.

Mitosis
Meiosis
Difference in Mitosis and Meiosis
Ileum
Functions of Cross Sections
- Serosa = outer layer of connective tissue providing very thin protective and supportive lining for the alimentary canal
- Muscularis Externa = consists of an outer layer of longitudinal muscle and an inner layer of circular muscle. The Muscle causes pendular movement and contration of the circular muscle cause localised constrictions which churn and mix the food bolus and is transported by the peristalic movements - Antagonistic Contractions
- Submucosa= largely comprised of connective tissueand contains many blood vessels and lymphatic vessel - crucial for transporting absorbed food products
- Muscularis Mucosa = thin layer of muscle that lies between the submucosa and the mucosa. It is important in moving the villi that are present in the mucosa. Increases the contact with the digested food in the gut lumen.- characteristic wafting movement of villi
- Mucosa= layer in contact with the food in the gut lumen. Highly Specialised with increase SA due to presence of Villi and Microvilli
Villus
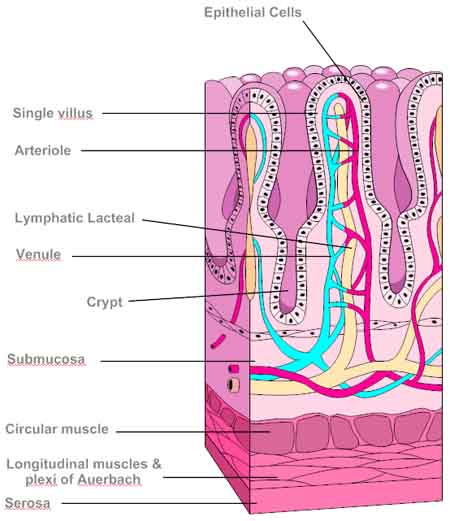
Cell Functions
Columnar Epilthelium Cells = column-shaped cells .On their free surfaces the cells have microvilli forming brush borders. Since digestive enzymes are bound to the membrane of the microvilli. This provides a hugh surface area for digestion and for the adsorption of the products of digestion. Some substances are taken up partly by diffusion and partly by active transport. Others are taken up by Pinocytosis. Numerous Mitocondria aids ATP energy production. Short lived cells.
Goblet Cells= secrete mucus . Protects the epilthelium from action of digestive enzymes and lubricates the lining as solid material is pushed along.
Villi= finger like projections which increase the SA for absorption of the digested products. The villi contain blood capillaries into which aminoacids and monosaccharides are absorbed and lacteals into which fats are absorbed.
Crypts of Lieberkuhn = intestinal glands are found at the base of the villi. Cells along the sides secrete mucus. Stem Cells lining the bottom of the crypts are in a state of continous division. New cells are continously being pushed up by the division of the cells deeper down.
Structure of Plant Cell
- Waxy Cuticle - Prevents water loss amd provides waterproofing
- Palisade Mesophyll Layer- photosynthesis . Rich in Chloroplasts
- Spongy Mesophyll Layer-gas exchange- intercellular spaces
- Xylem and Phloem - transport of ions and water moleucles
- Stomata - Allow gas exchange . Allows water vapour to diffuse out of the leaf


Comments
Report