Biological Molecules
- Created by: revision00001
- Created on: 04-01-23 10:38
Molecules
Monomers are single repeating units that make up polymers when joined together by cnodensation reaction, water is released from these reactions
Polymers can be broken down by hydrolysis reactions - bonds are broken using a water molecule

Sugars
General term for monosaccharides and disaccharides
Monomers that make up carbs are monosaccharides e.g glucose,fructose
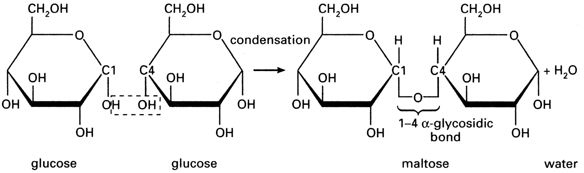
Glucose is a hexose sugar (6 carbon atoms), two types of glucose: alpha and beta
Disaccharides are formed from condensation of monosaccharides, a glycosidic bonds form between two monosaccharides and water molcule is released
Test for sugars (reducing)
enedict's Test
Reducing sugars:

Test for sugars (non-reducing)
Benedict's test
Non-reducing:

Polysaccharides
Formed when more than two monosaccharides are joined by condesation reactions. Functions of polysaccharides: starch, glycogen, cellulose
Starch: plants store excess glucose as starch as a mixture of amylose and amylopectin. Amylose long unbranched cahin alpha glucose, angles of bonds give coiled structure, means it's compact good for storage, Amylopectin long branched chain alpha glucose, side branches allow break down molecule getting to bonds more easily, glucose released quickly. Starch insoluble doesn't effect water potential.
Glycogen: animals store excess glucose as glycogen, similar structure to amylopectin due to numerous side branches, lots branches also means release glucsoe quickly, compact good for storage
Cellulose: long unbranched chains beta-glucose, when molecules bond form straight cellulose chains, chains linked by hydrogen bonds forming strong fibres called microfibrils, strong fibres means can support cell walls
Test for starch
Iodine test

Lipids
Two to know: triglycerides and phospholipids
triglycerides - one molecule of glycerol and three fatty acids, long tails of fatty acids are hydrophobic making them insoluble in water, fatty acids have same basic structure but saturated fatty acids din't have any double carbon-carbon bonds it is saturated with hydrogen, unsaturated fatty acids don't have any double carbon-carbon bonds causing the chain to kink, triglycerides are formed bycondensation reactions and an ester bond forms between two molecules releasing water molecule
phospholipids - in cell membranes the lipids are not triglycerides they're phospholipids, they are similar to triglycerides but one of fatty acids are replaced with phosphate group, the phosphate group is hydrophilic and fatty acid tails are hydrophobic
Properties of lipids
Triglyerides: mainly an energy store good for this because long tails contain lots energyreleased when broken down, insoluble in water don't effect water potential, hydrophobic tails group together on inside sheilded by glycerol heads so they bundle together and form droplets in water
Phospholipids: makeup bi-layer cell membranes, heads are hydrophilic pointing outwards and the hydrophobic tails face center formind double layer with heads facing out on either side, as centre is hydrophobic water-soluble molecules can't pass through easily, membrane acts as barrier to those substances
Test for lipids
Emulsion test
positive result shown by milky emulsion forming

Proteins
Monomer made of is amino acids, dipeptide from 2 joined, polypeptide from more than 2 joined, proteins made up of one or more polypeptide chains
Amino acids have general structure, there are 20 different amino acids difference is the R-group
Amino acids are joined by condensation reactions forming dipeptides and polypeptides
Peptide bonds form between amino acids
Protein structure
Primary structure - sequence of amino acids in polypeptide chain
Secondary - hydrogen bonds form between amino acids in the chain making it coil into either alpha helix or beta bleated sheets
Tertiary - coiled and folded chain is coiled more, more bonds form between different parts of the chain including hydrogen and ionic bonds, disulfide bridges form whenever two molecules of amino acid cytenine come close together (sulfur atom in one cytesine to sulfur in other)
Quaternary - some proteins made of several polypeptide chains held by bonds, quaternery structure is the way the polypeptide chains are assembled together, proteins final 3D structure
Protein shape and function
Shape determines function, specialised for each job
Enzymes: usually spherical-ish because of tights folding polypeptide chains, soluble often have roles in matabolism, other enzymes help to synthseise large molecules
Antibodies: involved in immune response, found in blood, made up of two light (short) and two heavy (long) polypeptide chains bonded together, have variable regions where amino acids vary greatly
Transport proteins: e.g channel proteins in membranes, hydrophobic and hydrophilic amino acids causing protein to fold up and form a channel, transport molecules and ions across membranes
Structural proteins: strong, long polypeptide chains lying parallel to each other with cross links between, examples include keratin and collagen, collagen has 3 polypeptide chains tightly coiled together making it strong, so makes it great to support tissues
Test for proteins
Biuret test:

Enzymes
Enzymes are biological catalysts, they are proteins and have an active site whith a specific shape this is where the complementary substrate binds, highly specific due to tertiary structure
They speed up reactions by lowering the activation energy needed for the reaction to take place speeding up rate of reaction
When a substrate fits into an enzymes active site it forns an enzyme-substrate complex
It lowers sctivation energy because:
- if two substrate molecules need to be joined, being attached ti enzyme hods them close together reducing any repulsion between molecules so bond easier
- if enzyme is catalysing a breakdown reaction, fitting into the active site outs a strain on bonds in the subtrate, so substrate molecules break uo moe easily
Enzyme action
'Lock and key model' : Enzmes only work with complementary substrates, early scientists developed the lock and key model to describe how they bond, it is the idea the substrate fits into the enzyme the same way a key would in a lock 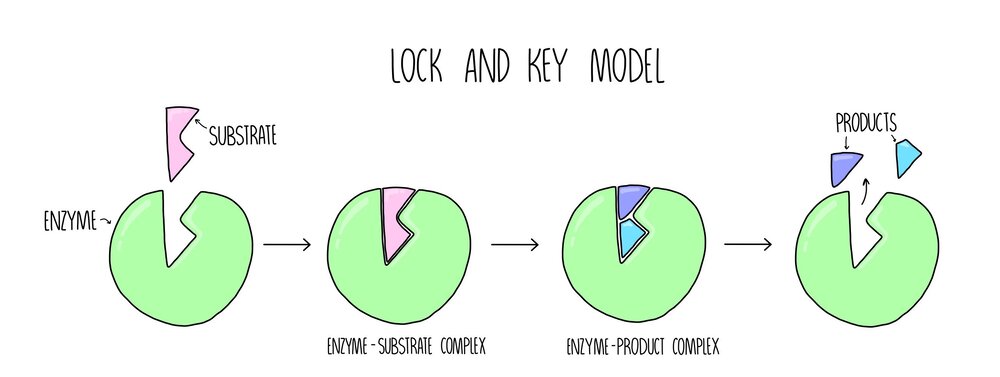
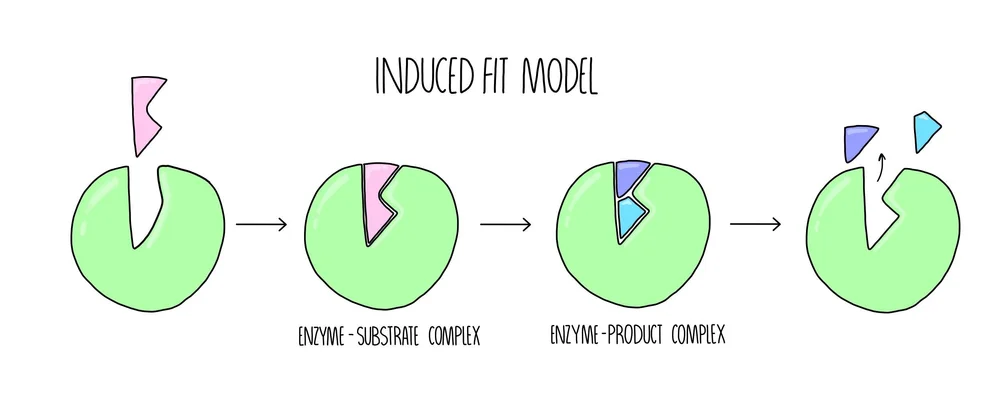
Soon realised this was too simplistic and actually the enzyme changes shape slightly when they bond to have a better more induced fit to the substrate... and so the
'Induced fit' model was invented: this exaplains why active sites are so specific, the substrate doesn't only have to fit the active site it has to make the active site change slightly the right way aswell, the slight change produces an induced fit
Enzyme structure
Properties are related to tertiary structure
If structure altered in any way shape of active site will change means substate won't fit and can no longer forn enzyme-substrate complex
Structure can be altered by pH or temperature
Primary structure determined by gene, if mutation occurs could change the tertiary structure of enzyme produced
Measuring enzyme activity
Two ways:
1) Measure how fast product made:
different molecules present at beginning and end of reaction, can measure amount of end product at different times during experiment and calculate rate of reaction
2) How fast substrate broken down:
to produce end products substrate molecules have to be used up, measuring anount of substrte molecules left at diferent times during experiment allows to calculate rate of reaction
Factors affecting enzyme activity
Temperature - increasing temp means more kinetic energy so molecules move faster there are more collisions and therfore more reactions take place, if temp gets too high reaction slows/stops because the higher temp casues bonds in the ezyne to break and the shape of the active site changes denaturing the enzyme so will no longer act as catylist
pH - all enzymes have optimum pH, above or below the optimum the H+ and OH- ions can disrupt ionic and hydrogen bonds holding the enzymes tertiary structure, enzyme becomes denatured and active site changes shape
Substrate concentration - higher the conc the faster the reaction as collision more likely between enzyme and substrate, after saturation point (when all active sites are taken up) enzymes have as much as they can cope with so adding any more substrate makes no difference
Enzyme concentration - the more enzymes there are in a solution the more likely they are to collide with a substrate molecule forming an E-S complex, increases rate of reaction to a point, if amount of substrate is limited comes a point when more than enough enzymes to deal with available substrate adding more enzyme has no further effect
Enzyme inhibitors
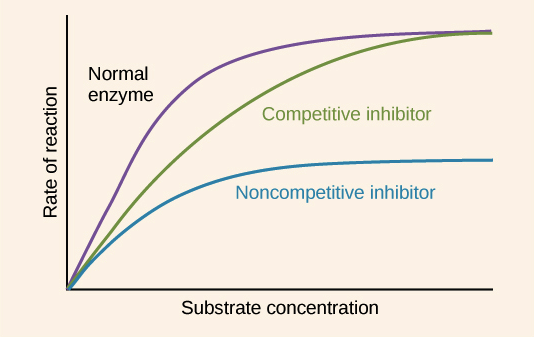
Enzyme inhibitors prevent reactions taking place, can be competitive or non-competitive
Competitive inhibitors: have similar shape to substrate molecules and compete with substrate to bind to the active site but no reaction takes place, they block active site preventing substrate molecules binding, if high conc of inhibitor they take up nearly all active sites but if higher conc of substrate increases chance of binding to enzyme before inhibitor increase, increase sub conc inceases rate reaction to a point
Non-competitive inhibitors: bind to enzyme away from active site but causes active site to change so substrate can no longer bond, don't compete with substrate because they are a different shape, increaseing sub conc won't have a difference, enzyme activity will still be inhibited
DNA and RNA
Both types of nucleic acid, DNA stores genetic info, RNA transfers DNA to ribososmes where it is read in process called translation ready for protein synthesis
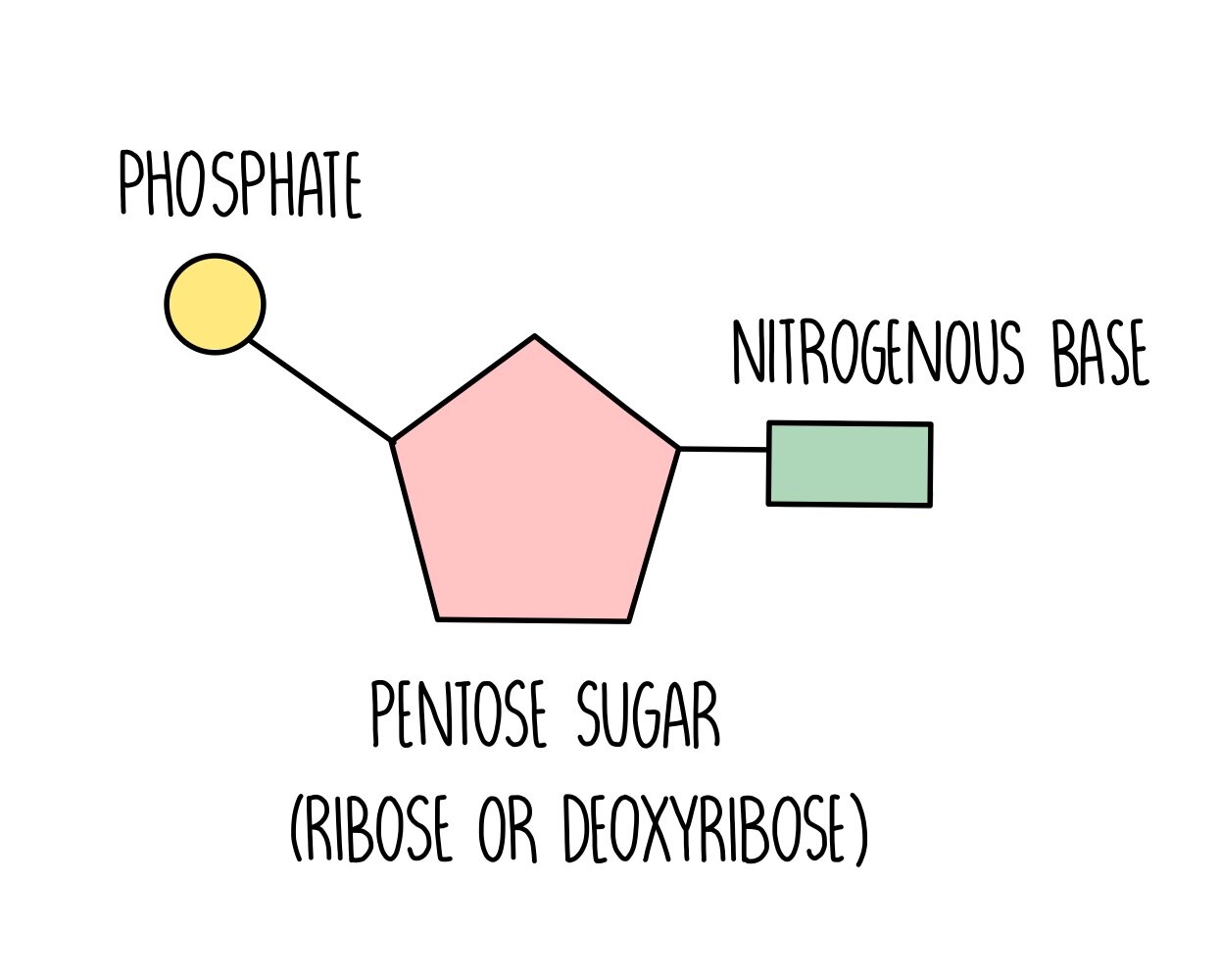
Nuceotide structure - DNA and RNA are polymers of nucleotides, a nucleotide is a biological molecule containing a pentose sugar, a nitrogen containing base, and a phosphate group
Polynucleotide structure - manyy neucleotides join together forming a polynucleotide chain/strand, a phosphodiester bond forms between the phosphate group of one nucleotide and the sugar of another, the chain of phosphates and sugars is known as sugar-phosphate backbone
DNA structure
Double helix structure, two separate polynucleotide strands run antiparallel and twist around each other to form double helix shape.
DNA made of pentose sugar deoxyribose, a phosphate group and an inorganic notrogen containing base. All same phosphate and sugar but base varies, 4 possible bases: adenine, thymine, cytosine and guanine
Two nucleotide strands join together by hydrogen bonds between complementary base pairs. adenine always to thymine and cytosine always to guanine. Always equal amounts of A and T with two hydrogen bonds between and equal amounts of C and G with three hydrogen bonds between.
RNA structure
Similar to DNA but differsin 4 ways:
- Sugar in RNA is ribose
- Uracil replaces thymine as base
- Nucleotides form single polynucleotide not a double
- RNA strands much shorted than DNA polynucleotides
DNA replication
DNA copies itself before division so each new cell has full amount of DNA, this called semi-conservative replication because half DNA in new cell is from original, meaning theere is genetic continuity between generations
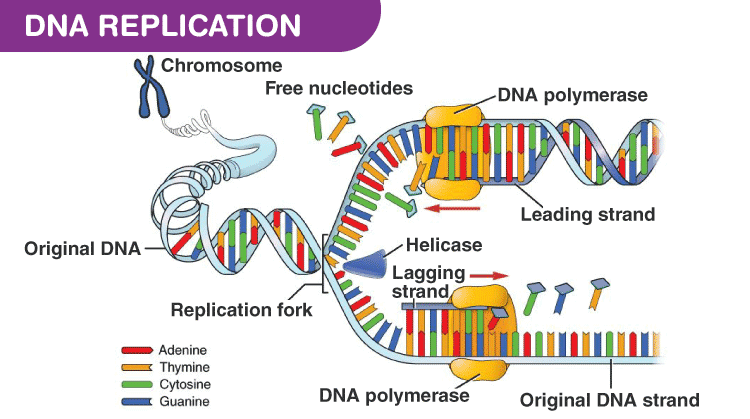
DNA helicase breaks down hydrogen bonds between bases making helix unwind, each strand acts as a template for new strand, complementary base pairing means free floating DNA attach to original template, condensation reactions join nucleotides of new strands together - catalysed by DNA polymerase, hydrogen bonds form between bases, each new molecule contains one strand of original DNA and one new strand
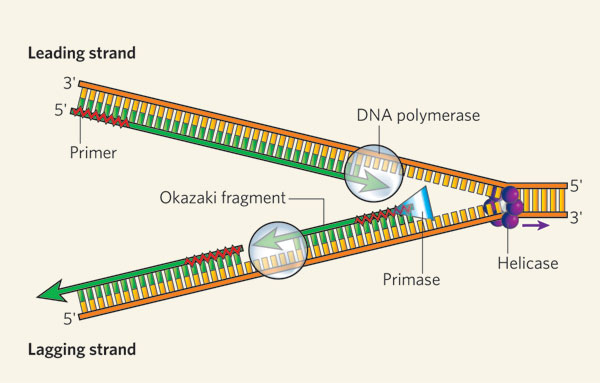
DNA polymerase
Each end of strand slightly different, one is 3' end and the other 5' end
During replication active site of polymerase only complementary to 3' end so can only add nucleotides at the 3' end
Means new strand made in 5' to 3' end direction and polymerase moves down template strand in 3' to 5' direction
because strands are antiparallel, DNA polymerase working on strand works in opposite direction to polymerase working on other stand (two white bubbles on image show this action)
Evidence for semi-conservative replication
Meselson and Stahl's experiment
1) Two bacteria samples grown in broth: one containing light nitrogen one containing heavy nitrogen so it eventually became part of their DNA
2) Sample of each bacteria was taken and spun in centrifuge, heavy nitrogen settled lower than light nitrogen
3) Both bacteria put in light nitrogen broth and left for one ound of DNA replication and another sanmple was taken and spun in centrifuge
4) If replication was conservative original heavy DNA would settle at bottom and light DNA would dettle further up. If replication semi-conservative new DNA would settle somwehere between the two, as it contains one strand of old heavy nitrogen DNA and one strand of new light nitrogen DNA
6) The DNA settled in the middle showing it was a mix of light and heavy DNA
Once confirmed semi-conservative replication took place, other scientists did experiments to show it was universal replicaion method for all living things
ATP
Made from nucleotide base adenine, ribose sugar and three phosphate groups
Energy is not directly from glucose, energy released from glucose is used to make ATP, once made energy is stored in the high energy bonds between phosphate groups and released via hydrolysis reactions
When energy needed ATP broken down into ADP +Pi by hydrolysis reaction catalysed by ATP hydrolase, energy released from phosphate bond. Released inoranic phosphate can be added to another compound to make it more reactive (phosphorylation). ATP can be resynthesised in condensation reaction between ADP and Pi catalysed by ATP synthesase

Water
Essential for life
About 80% of cell's components
- A metaboloite in lots of metabolic reactions including condensation and hydrolysis reactions
- Solvent means some substances dissolve in it, most metabolic reactions take place in solution so water is essential
- Helps with temporature control because of high latent heat of vaporisation and high specific heat capactiy
- Water molecules very cohesive which helps transport in organisms especially plants
Water structure
Polarity - molecule of water is one atom oxygen joined to two atoms of hydrogen by shared electrons, because the shared negative hydrogen electrons are pulled towards oxygen atom the other side of each hydrogen left with slight positive charge, unshared negative electrons on oxygen atom give it slight negative charge, makes polar slight neagtive charge on one side and negative on the other
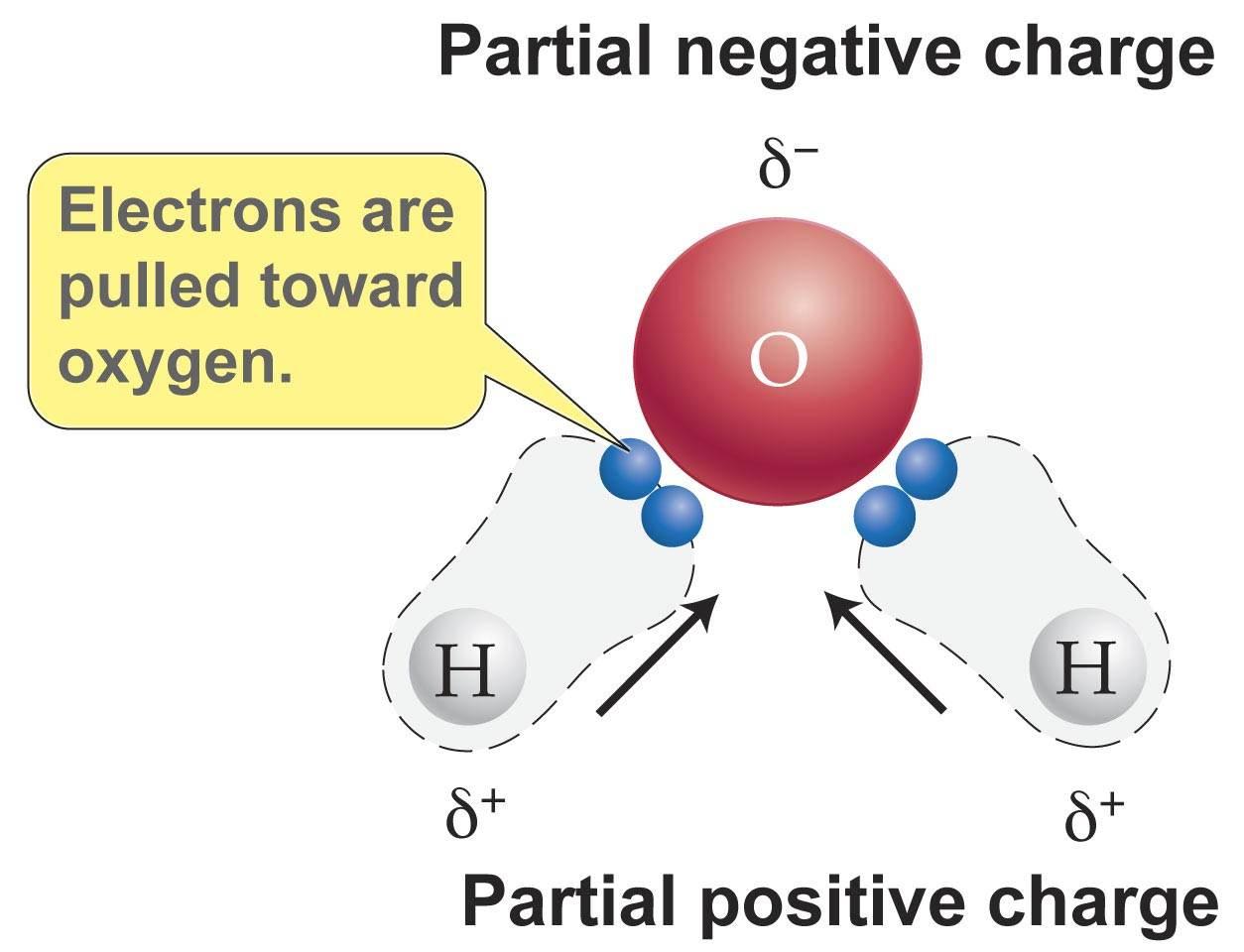
Hydrogen bonding - weak bonds between a slightly positively charged hydrogen atom in one molecule and a slightly negatively charged atom in another molecule, hydrogen bonds form between water molecules because the slightly negatively charged oxygen atoms attract the slightly positively charged hydrogen atoms of other water molecules
Water properties
Important metabolite: many metabolic reactions involve condensation or hydrolysis reaction, hydrolysis involves water to break bonds, condensations releases molecule when bond formed, e.g ATP released hydrolysis, amino acids form proteins by condensation
Good solvent: lots substances in biological reactions are ionic, because water is polar slightly positively charged end water attracted to negatively charged atom, slightly negatively charged end water attracted to positively charged ion, means ions totally surrounded by water molecules = dissolve, living organisms take up useful substances dissolved in water and these dissolved substances can be transported around organism body
Water properties 2
High latent heat of vaporisation: water vaporises when hydrogen bonds holding water molecules together are broken, molecules on surface of water escape into air as gas, takes lots energy to break hydrogen bonds between water, lots energy used up when water evaporates so high latent heat of vaporisation, lots of heat used change water to gas, useful for organisms can use water loss to cool down without loosing too much water, when evapourates carries heat energy from surface which cools surface to help lower temperature
Buffer changes in temp: hudrogen bonds give high specific heat capacity, when heated lots energy used to break bonds means less energy available to actually increase temp so has specific heat capacity, takes lot energy to heat up, useful for living organisms means water doesn't experience rapid temp changes, makes good habitat because temp underwater likely more stable on land, water inside organisms remains stable temp helps to maintain constant internal body temp
Very cohesive: attraction between molecules of same type, water molecules very cohesive because they are polar, strong cohesion helps water flow making it good transport substances, e.g it's how water travels in columns up the xylem, strong cohesion means water has high surface tension when comes into contact with air which is the reason sweat droplets form which evaporate from skin to cool organism down
Inorganic ions
Ion is an atom or group of atoms that has an electrical charge, an ion with a positive charge is called a cation
An inorganic ion is one that doesn't contain carbon
There are inorganic ions in solution in the cytoplasm of cells and in bodily fluids of organisms
Each ion has a specific role depeding on its properties
An ions role depends on whether it is found in high or low concentrations
e.g iron ions in haemoglobin
in the red blood cells, made up of 4 different polypeptide chains each with an iron ion in the centre, it's the iron that actually binds to the oxygen in haemoglobin so it's a pretty key componenet, when oxygen is bound the Fe2+ ion temporarily becomes an Fe3+ ion unitl oxygen is released


Comments
No comments have yet been made