Polymers
- Created by: Frankie Rushton-Smith
- Created on: 04-04-18 19:37
Fullscreen
11.1 Addition polymerisation
- Hydorcarbons are the main fuels for cars and the chemicals from hydrocarbons are most importantly used to make polymers
- Plastics are made from very large, covalently bonded molecules called polymers
- Monomers are the small molecules that bond together to make huge molecules called polymers
- Ethene can be used to make polyethene which is a useful plastic as it's v. strong and easy to shape. It's transparent unless colours added to it. Eg.s = carrier bags, cling film, dustbins
- ethene monomers --> poly(ethene)
- Propene is an alkene used to make polypropene, which is a strong, tough plastic. E.g. ropes, carpets, milk crates
- propene monomers --> poly(propene)
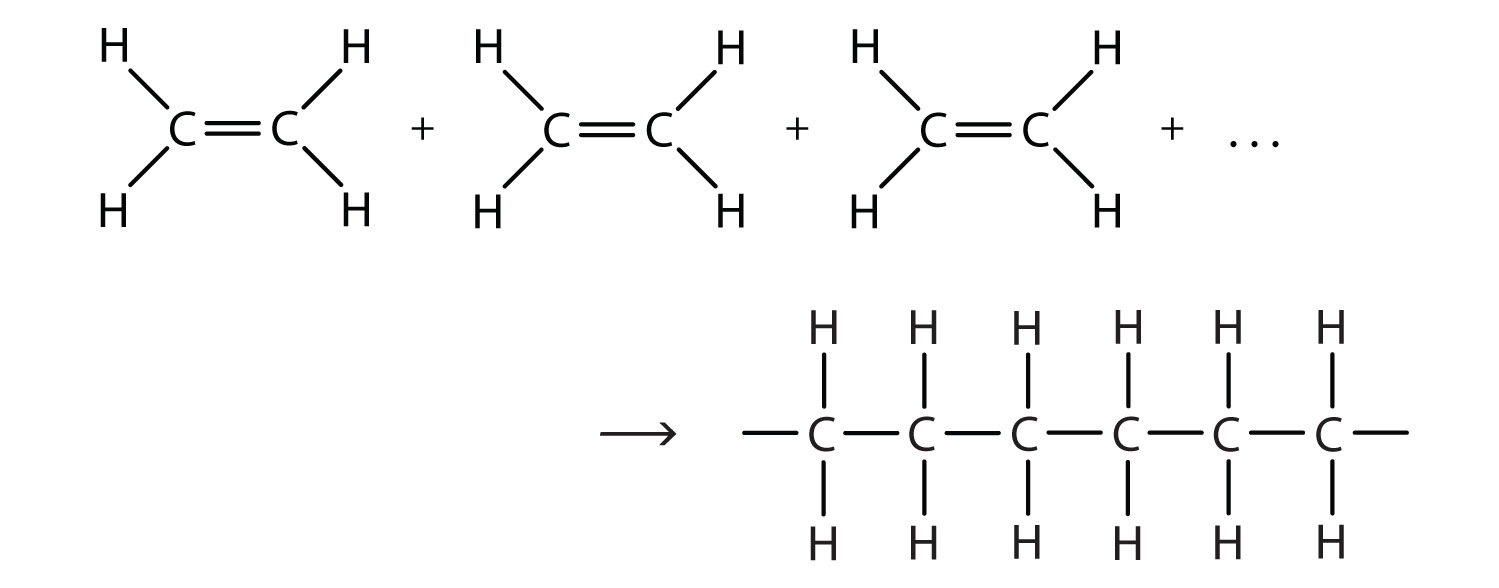
- When alkene molecules join, the double covalent bond between the carbon atoms 'opens up' and is replaced by a single carbon-carbon bond between the 2 carbon atoms. The polymer chains formed, make up the 'backbone' of 1000s of carbon atoms -- this reaction is addition polymerisation and the polymer made is called the addition polymer
- Polymers are made of repeating patterns of atoms
11.2 Condensation polymerisation
- Common polymers = nylon and polyester and nylon is strong, lightweight, easy to care for, non-absorbant, dry quickly, few wrinkles after being washed. Polyester and nylon are condensation polymers, not addition polymers
- In addition polymerisation there's one product made but in condensation polymerisation there are 2 products made. Main product = polymer and there's also a small molecule given off - usually either water or hydrogen
- addition polymerisation --> the addition polymer
- condensation polymerisation --> the condensation polymer + a small molecule
- Monomers in addition polymerisation are often the same alkene but in condensation polymerisation there are usually 2 different monomers used. One monomer will have a functional group at both ends of its molecule and the other will have a…
Comments
No comments have yet been made