Topic one - Principle of chemistry
- Created by: DANIELLEISGREAT
- Created on: 16-02-17 18:11
Solids, liquids and gases:
- Solids have a fixed volume and shape
- Liquids don't change volume but have no definite shape, tey take up the shap of the container they are in
- Gas has no fixed volume or shape, they spread to fill their container
Diffusion:
When potassium managnate(VII) dissolves in water the water slowly all turns purple because of the random motion of particles in a liquid the purple colour eventually evenly spreads out.
Another experiment that can be used to show diffusion is bromine gas and air, you fill half a gas jar with bromine and half with air then when you remove the lids seperating the two gases then if you leave it for long enough , because of kinetic theory, the particles of bromine gas move around randomly (random motion) so both jars are filled with bromine.
Mixtures and compounds:
Mixture- Two or more substances that are combined without a chemical reaction soo can be seperated easily
Compound - When atoms join together in a chemical reaction , the compound has very different properties than the elements they derived from and they can only be seperated through another chemical reaction.
Distillation seperates a liquid and a solid, one example being seperating salt and water.
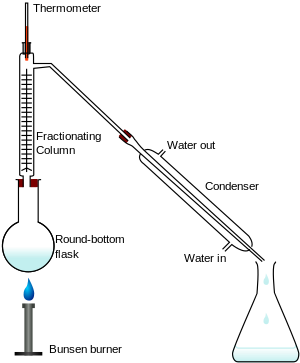
Fractional distillation seperates mixtures of liquids and relies on how liquids boil at different temperatures.
Filtration also serperates solids from liquids , the mixture passes through filter paper and the liquid goes through but the solid is left behind.
Crystallisation , when a solid is dissolved in water , you can just evaporate the water so only the solid is left behind.
Chromotography paper:
Chromotography can be used to seperate a mixture of solids that are soluble, it is often used on inks and dyes. To seperate the different dyes in an ink a spot of ink must be put at the bottom of the chromatogograhy paper , then make sure some of the paper , below the ink, is in water. As the water spreads throuought the paper the dyes are carried with the water and they begin to seperate , this is because different dyes have different solubilities and are carried at different speeds along the paper.
Atomic structure:
Atoms are made of smaller , sub-atmoic particles , the main three ones are protons, neutrons and electrons.
Because the protons in the nucleus have a positive charge and the neutrons have no charge the nucleus is postitivley charged , which keeps the elctrons orbiting it in their shells as they are negaticley chraged so they attract.
Isotopes:
Isotopes are atoms which have the same number of protons as elctrons but have a different number of neutrons. …

Comments
No comments have yet been made