Alevel chemistry: Aromatic compounds
- Created by: Rachaelg
- Created on: 15-07-20 15:30
Fullscreen
Aromatic compounds and reactions of Benzene
Benzene is a hydrocarbon compound with a molecular formula of C6H6.
The original model for the structure of benzene - Kekules' model stated that benzene was formed of 3 alternating double bonds in a ring of the six carbon atoms. The model was proven wrong due to the following evidence:
- benzene doesn't undergo electrophilic addition- suggesting there aren't any double bonds
- bond lengths in benzene are all the same - shorter than a single bond and longer than a double bond
- The enthalpy change of hydrogenation was less than expected
The new delocalised model proposes that benzene is formed of six carbon atoms joined with single sigma bonds and the p orbital electrons are spread above and below the plain in a delocalised pi system.
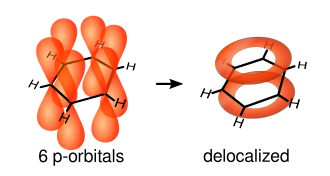
Naming compounds with benzene rings:
When naming compounds with only one constituent group a prefix is used such as ethyl or chloro and the word benzene is…
Comments
No comments have yet been made