Alevel chemistry: Amines
- Created by: Rachaelg
- Created on: 13-06-20 11:54
Amines
Amines are oragnic compournds that has a nitrogen atom bonded to a carbon chain or ring. The N is derived from the compound ammonia- NH3.
When the nitrogen atom is bonded to a straight carbon ring the compound is known as an aliphatic amine but when bonded directly to an aromatic ring it is known as an aromatic amine.
Amines can be classified as primary secondary or tertiary. When the bonding nitrogen is attached to 1 carbon it is primary, when there are 2 carbon atoms bonded it is secondary. Remember nitrogen normally forms 3 bonds and when not bonding to carbon the other bonds will usually be taken up by hydrogen atoms. 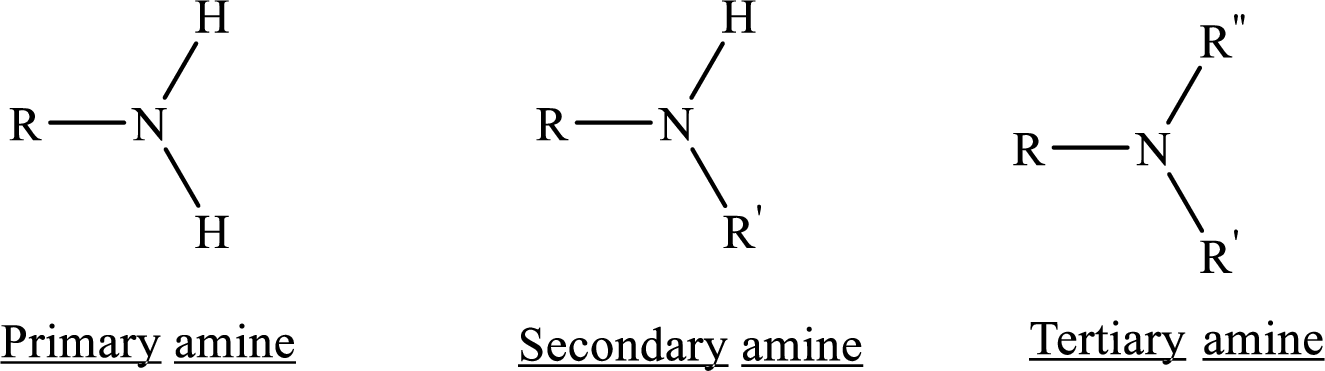
Amines can sometimes behave as bases: they can accept a proton to form 4 bonds one of them a dative covalent bond with hydrogen. This is due to the lone pair present on the nitrogen atom.
This property also means amines can react with acids in a…
Comments
No comments have yet been made