Water and Carbohydrates
- Created by: Jen Purbrook
- Created on: 28-04-17 14:37
Overview of Biological Molecules
One important class of molecules is carbohydrates.
The smallest carbohydrate molecules are called sugars. They are made up of several carbon, hydrogen and oxygen atoms all joined together.
Sugar molecules can join together and form long chains producing huge molecules such as starch, glycogen and cellulose. These are all carbohydrates.
Protein molecules are made up of long chains of smaller molecules --> called amino acids. Thes also include nitrogen atoms
Nucleotides are molecules such as DNA and RNA.
Lipids are also known as fats
Monomers and Polymers
The largest molecules are made by joining together long chains of smaller molecules --> starch is made by joining together very long chains of glucose molecules.
The glucose molecules molecules are monomers and the long chain of them joined together forms a polymer
Protein molecules are polymers --> the amino acids are monomers
Nucleotides form polymers, joining together in long chains to form polynucleotides
Structure of Water Molecules
Water molecules are made up of two hydrogen atoms joined by a covalent bond to a single oxygen atom, so the formula is H2O.
Water molecules are very small compared to other molecules.

Covalent bonds are very strong bonds, formed when two atoms share electrons.
It is very hard to split the H and O atoms apart (you need a lot of energy)
Water Molecules

Water Helps Keep Organisms Cool
In a body of water, not all of the water molecules are moving at the same speed so some molecules have a more kinetic energy than others. Some molecules will have enough kinetic energy to become a gas. This is called evaporation.
As these molecules are lost from the water, their energy goes with them. As more molecules are evaporating the average kinetic energy of the molecules left behind in the water gradually decreases. The water then cools down.
The energy that is lost from the liquid water as it evaporates is called latent heat of vaporisation. Water has a high latent heat of vaporisation, so the evaporation of quite small quantities of water has a large cooling effect.
We make use of this when we sweat. Sweat lies on the skin surface and evaporates. As it evaporates, the skin surface is cooled.
Plants are cooled too when water evaporates from their leaves in transpiration.
Hydrogen Bonds
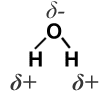
Atoms contain electrons which have a small negative charge.
In a water molecule, the electrons in the chemical bond are not shared equally between the O and the H atoms. This means that the O atom has a tiny negative charge and each H atom has a tiny positive charge. The water molecule is said to have a dipole.
Although its overall charge is 0, it has a small + charge in some places and a small - charge in another.
These small charges are written as delta minus and delta plus
Negative charges and positive charges are attracted to each other. This means that the negative charge on one water molecule is attracted to another water molecule with a positive charge. This is called a hydrogen bond.
Hydrogen bonds are weaker than covalent bonds.
Water as a Solvent
A solvent in which other substances (solutes) can dissolve and water is an excellent solvent. Many different substances are able to dissolve in water --> because of hydrogen bonds.
Water can dissolve smaller molecules such as soduim chloride (salt) and quite large molecules such as sugars.
When substances are dissolved in water, their molecules or ions are free to move around and to react with other molecules or ions that are also in the solution. The metabolic reactions that take place in your body only take place because the reactants are dissolved in water.
Water's solvent properties are also important in transporting substances around the body. Blood plasma is mostly water and it carries a huge range of different substances in solution --> glucose, vitamins, urea and carbon dioxide.
We also use water to dissolve substances to be excreted from the body, especially urea. Urine is a solution of urea and other substances in water
Water Molecules Stay Together
The movement of a whole mass of together is an example of mass flow.
In plants, water moves up xylem vessels as continuous stream by mass flow.
The way in water molecules tends to 'stick' together is called cohesion.
Normally, a water molecule is attracted to water molecules on all of its sides. But at the surface of a body of water, the top layer of water molecules do not have any water molecules above them, so the net attraction is downwards.
This provides surface tension, making the water behave as though there is a thin 'skin' where it meets air. Surface tension allows small animals to walk on the water surface --> pond skaters
Carbohydrates
All carbohydrates contain the elements carbon, hydrogen and oxygen.
The H and O atoms are generally in ratio of 2:1, giving carbohydrates the general formula CH2O.
There are three basic types of carbohydrates:
- monosaccharides
- disaccharides
- polysaccharides
Monosaccharides
These are simple sugars --> small molecules that dissolve in water and taste sweet.
These are the monomers from which all larger carbohydrates are made.
Glucose is a monosaccharide. Five C atoms form a ring. It can exist in two different forms, depending on the hydrogen and hydroxyll (-OH) groups on C atom 1. These are alpha glucose and beta glucose. They are isomers of each other = their molecules are made of the same numbers of C, H and O but the atoms are arranged differently.
Fructose and Galactose are isomers of each other. They have the formula C6H12O6 ,but they have slightly different properties of one another.
All monosaccharides are soluble in water.
Alpha Glucose and Beta Glucose

Fructose and Galactose

Disaccharides
Disaccharides have molecules made of two monosaccharides joined together. They are soluble in water and taste sweet.
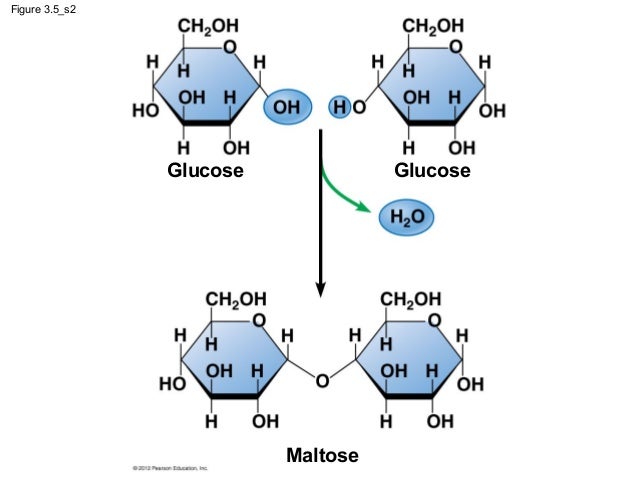
Examples include maltose (2 glucose molecules), sucrose (1 glucose molecule and 1 fructose molecule) and lactose (1 glucose molecule and 1 galactose molecule).
Maltose is found in germinating seeds, sucrose is the sugar we add to drinks or in cooking and lactose is found in milk.
Maltose and sucrose are both formed from condensation reactions and a water molecule is formed. The bond that is formed between the two sugar molecules is called a gylcosidic bond.
Disaccharides can be broken down into their component monosaccharides. The reactions happen in reverse. Water molecules must be used so it is called a hydrolisis reaction.
Formation of Maltose
Formation of Sucrose
Polysaccharides
Polysaccharides are giant molecules made up of many monosaccharide molecules joined together by condensation reactions. Polysaccharides are insoluble, because their molecules are so large and cannot spread out in between water molecules as smaller molecules do.
Starch is a polysaccharide. Starch molecules are compact, coiled and branched making them ideal 'energy' stores.
Starch is a polymer (a molecule made up of repeating units, like links in a chain). The units are called monomers, in this case they are alpha glucose molecules. These bonds produce twisted chains of monomers. The coiled and brached chains of starch molecules give them a compact shape which makes them the idead 'energy' storage compounds.
When required they can easily be hydrolised to individual glucose molecules by enzymes that break the alpha- 1,4 glycosidic bonds. The glucose can then be used in respiration to release energy.
Polysaccharides 2
Another advantage of using starch as a storage compound is that its large molecules cannot cross membranes. Because its insoluble, starch doesn't have any osmotic effects on the cell, as glucose would. Storing glucose would tend to move into the cell by osmosis.Starch is the major storage carbohydrate in plants and thus the major carb in our diet.
Animals use glycogen as their storage carbohydrate. Glycogen molecules are very similar to starch molecules - they are made up of alpha-glucose molecules joines by 1,4 and 1,6 glycosidic bonds - but they have more branches than starch.
Glucose monomers are joined in a different way to form cellulose. Cellulose is the most common polysaccharide, because it is the major substance from which plant cell walls are made.
Cellulose molecules are made up of long chains of beta-glucose molecules, all joined through 1-4 glycosidic bonds. There are no branches --> this produces long, straight molecules that lie side by side. Cellulose molecules form huge numbers of H bonds with all other cellulose molecules lying alonside them, forming groups of parallel molecule call microfibrils. They are difficult to digest and have great strength. Cellulose is an excellentstructural substance rather than an energy storage substance like starch and glycogen.
Biochemical Test for Starch
To test for starch, add iodine in potassium iodide solution to the substance. A blue-black colour indicates the presence of starch.
Biochemical Test for Sugars
Tests for sugars can distinguish two groups if sugars: the reducing sugars and the non-reducing sugars
To test for reducing sugars, we add Benedict's solution to the substance, then heat the mixture in a water. An orange-red precipitate indicates the presence of a reducing sugar.

Related discussions on The Student Room
- Do I need to know how to draw structures for carbohydrates? (AQA A Level Bio) »
- Mark my AS level bio essay pls »
- How to answer 6-mark questions in A-Level Biology? »
- How to lose face fat? »
- Calvin Cycle »
- Paper 3 AQA a Level biology »
- I really need help!!!!!!!! *Biology* »
- exams 2022 »
- Alevel biology understanding »
- Animal Transport Notes OCR A Level »
Comments
No comments have yet been made