Permanent dipole is when one atom is more electronegative than the other atom.
Why?
Because it has more protons and electrons and therefore a bigger attraction will happen as opposed to another atom who doesnt have many protons and electrrons.
E.g H-Cl
But whenever the dipoles are symetrical to each other e.g CH4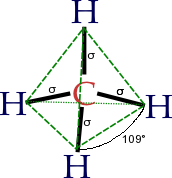 The dipoles act in different directions and cancel each other out!
The dipoles act in different directions and cancel each other out!
Comments
No comments have yet been made