Topic 18A: Arenes - Benzene
For New Spec Edexcel (2015) A Level. This is topic 17. in the textbook. (I have similar resources for Topic 17 and Topic 18B in organic chemistry)
- Chemistry
- ArenesBonding & shapesFunctional GroupsBenzeneMechanismsAcids, bases and saltsIntermolecular forces
- A2/A-level
- Edexcel
- Created by: LouiseG
- Created on: 07-03-17 10:36
Developing the Benzene Model - Kekule
The bonding and structure of benzene was a mystery for a long time after it was first isolated and identified as a compound. One of the key stages in deciphering its structure was the discovery of its molecular formula, C6H6.
The molecular formula of benzene suggests it is highly unsaturated (alkanes, for example, have a CnH2n+2 general formula, and alkenes CnH2n - whilst benzene has equal numbers of carbons to hydrogens). It was deduced by German chemist Kekule that this was in keeping with the cyclic alkenes, which have fewer hydrogens per carbon than their straight-chained alternatives. He proposed that benzene has a ring structure with 3 double bonds:
This is known as the Kekule structure, and would be called by IUPAC nomenclature cyclohex-1,3,5-ene.
Problems with Kekule Model
However, some odd aspects soon arose in benzene's chemical behaviour which were not easily explained by the Kekule model. They provided the evidence to prove the Kekule model was not entirely accurate.
1. Benzene doesn't react with bromine water
The structure of benzene proposed by Kekule suggests it is an alkene, therefore should readily undergo electrophilic addition reactions - for example with bromine water. However, benzene shows up negative for the common test for alkenes: it doesn't decolourise bromine water. This means benzene is resistant to addition reactions, suggesting it has a more stable structure that Kekule's model can explain.
2. Identical Isomers?
Kekule's model suggests that there should be two different molecules (position isomers) with adjacent bromines in dibromobenzene, with the bromine groups on 1,2- and 1,6- . However, chemical tests revealed they were, in fact, identical. This does not fit with Kekule's structure.
More Problems with Kekule Model
3. The hydration of benzene is less exothermic than expected
If benzene had a structure like cyclohexene, but with two more double bonds, it would make sense to assume the hydration of benzene (breaking the double bonds and adding hydrogen, to form cyclohexane) would be 3x more exothermic than for cyclohexene. However, the real value is less exothermic than expected, suggesting that benzene was far more kinetically stable than the proposed structure.
Another Problem with Kekule Model!
4. X-Ray crystallography showed benzene didn't contain C=C and C-C bonds
If benzene was made of 3 C=C and 3 C-C bonds, you'd expect to measure 2 different carbon-carbon bond lengths (which is done with X-Ray crystallography): the shorter double bond and longer single bond. Furthermore, you'd expect the hexagon of benzene to be slightly distorted due to 3 "sides" being shorter. However, benzene was found to have only one carbon-carbon bond length, and to be a perfectly symmetrical, regular hexagon. The bond lengths also fell between that of a double bond and single bond, indicating that benzene has bonds which are not quite of either type.
The 4 observations lead to the modern model of benzene. It is now suggested that the p-orbitals of each of the 6 carbons overlap to form one, continuous pi-bond "ring" above and below the carbon plane. This is represented in skeletal form as a circle in a hexagon: 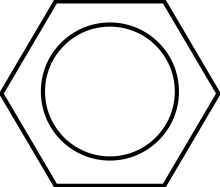
Modern Structure of Benzene
So how does the delocalised model explain the four issues of benzene?
1. Resistant to bromination - Benzene has a delocalised pi bond ring compared to an alkene's "localised" pi-bond in its double bond. The delocalised structure is energetically stable enough that benzene would usually rather undergo substitution reactions (replace an H, keep the ring) than addition (the ring is broken).
2.Identical "isomers" - The delocalised ring means benzene has rotational symmetry. So two bromines next to each other (i.e. positions 1 and 2 or 1 and 6) are identical molecules, compared to the Kekule model where the position of the double bond would suggest the two forms are different.
3.Lower hydrogenation enthalpy - Benzene's ring structure is more kinetically stable than the 3 double bond model, therefore it releases less energy on hydrogenation.
4. Identical bond lengths - All the carbon-carbon bonds in benzene are identical, they between single and double bonds.So all the bonds are the same length.
Reactions of Benzene
Benzene, like other organic compounds, can be combusted:
C6H6 + 7.5 O2 ----> 6CO2 + 3H2O
Benzene burns with a smoky flame, compared to the much bluer, "clean" flame of short-chained alkanes and alcohols like ethanol. This is as benzene has a high carbon to hydrogen ratio, meaning there are many carbon atoms per hydrogen (1:1 ratio compared to 2:6 in ethane, for example) which results in carbon particulates forming on combustion (even if the conditions aren't particularly oxygen-deprived). A similar effect is seen with other long-chained hydrocarbons and organic compounds, and alkenes (which are also lacking in hydrogens).
Electrophilic Substitution
So far, you will have met electrophilic addition (how alkenes react with molecules such as HBr, when an electrophile attacks the double bond) and nucleophilic substitution (when the halogen in a haloalkane is replaced by a nucleophile like OH-) but not electrophilic substitution. This is the reaction that benzene undergoes as it prefers not to break its stable delocalised ring, but instead replaces one or more of the H's with species that are attracted to the electron-rich ring. These reactions are quite easy to work out as they all have the same general mechanism:
An electrophile (here, A+) approaches the benzene ring, which breaks open to form an unstable carbocation intermediate. Notice that the H here is just the H from the benzene ring, displayed to show how it behaves in the reaction, NOT another species that's become attached. This hydrogen then donates its electron back to the benzene ring, so that the delocalised pi-bond can reform. This leaves a H+ ion, and the electrophile now added to benzene in its place.
Bromination
Here is an example, with Bromination, which occurs under anhydrous conditions in the presence of a FeBr3 (Iron(III) bromide) catalyst.
The catalyst is needed to generate the electrophile, which it does by taking one bromine out of the diatomic molecule to leave a Br+ (which is called the bromonium ion:) FeBr3 + Br2 ---> FeBr4- + Br+
The bromonium ion can now attack benzene as seen in the general formula:
Lastly, as we know that catalysts need to be reformed in a reaction, FeBr3 is retrieved by reaction with the H+ ion: FeBr4- + H+ ---> FeBr3 + HBr
To generate the catalyst, iron filings need only be added to the reaction vessel with the bromine. This is as the iron will react to form FeBr3 in situ, and then go on to react with more bromine and form the electrophile.
Nitration
The nitration of benzene is an important reaction as it produced nitrobenzene, used in pharmaceuticals and explosives and to synthesise other organics like phenylamine. Nitrobenzene isn't benzene with nitrogen attached, but an NO2 group (not to be confused with nitrogen dioxide, the electrophile is NO2+).The reagents needed to produce the attacking electrophile are concentrated sulfuric acid (the catalyst) and nitric acid (a reagent):
H2SO4 + HNO3 ---> NO2+ +HSO4- + H2O
The electrophile then attacks, as before:
And the catalyst is reformed:
HSO4 - + H+ ---> H2SO4
Friedel-Crafts Reactions
Friedel-Crafts reactions allow a variety of hydrocarbon groups to be added to benzene through the use of a halogen carrier, AlCl3. A halogen carrier does what it says: it "takes" a halogen off a reactant species to create an electrophile, which can then react with benzene in the same way as seen in the last few slides (attack to form an unstable intermediate, reforming of the ring by the removal of hydrogen). The general equation is:
1. X-E + AlCl3 ---> AlCl3X- + E+
2.
3. H+ + AlCl3X- ---> AlCl3 + HX
In general, X-E + Benzene ----> Benzene with E substituted + HX
where X is a halogen.
You can see that AlCl3 is reformed (it is a catalyst) so you can think of it as "carrying" the halogen X for the duration of the reaction and then giving it back up at the end.
Alkylation
You can tell from the last slide that any reactant for the Friedel-Crafts to work would have to contain a halogen. For alkylation (the addition of an alkyl group, CnH2n+1) this means it needs to be a haloalkane. For example, if you wanted to make methylbenzene, you could start off with bromomethane or chloromethane, for example:
CH3Br + AlCl3 ---> AlCl3Br- + CH3+
Followed by the electrophilic attack of the methyl ion, and finally the reaction of the hydrogen ion with the AlCl3Br- to form HBr and reform the catalyst.

Acylation
The same principle applies to acylation (the addition of the acyl group, which has the carbonyl functional group -COR), with an acyl chloride used as the initial reagent.
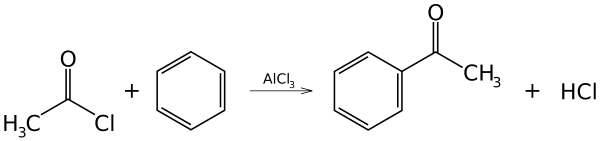
The same reaction occurs.
(Notice you may have seen throughout these slides benzene represented by both the delocalised ring and Kekule model. For your mechanisms, you are allowed to use either. The only difference is, if you choose to use the Kekule model you must show your electron movement in electrophilic attack from a double bond, not a single bond. I would use the delocalised model throughout because it's more accurate and easier to draw the mechanism). Also, a note on the intermediate: The "horseshoe" broken-circle in the intermediate should cover about 4 of the 6 carbons, and the open end (where the delocalisation breaks) should always face the carbon where the electrophile is being attached.
Phenol
Phenol is an organic compound with an -OH group attached to the benzene ring. It is the simplest aromatic alcohol. Phenol is interesting as it is far more reactive than benzene. This is as the lone pair on the oxygen overlaps with the delocalised ring and thus increases the electron-density of the ring. This is sometimes called activation, as it activates the stable benzene ring to undergo more reactions, and more easily (e.g., reactions may happen without a catalyst and at room temperature). This is as it attracts electrophiles even better than benzene.
For example, the bromination of phenol doesn't need a catalyst like benzene's FeBr3. This is as the bromine molecule self-ionises as it approaches the electron-dense ring, splitting up into Br- and the Br+ electrophile. The reaction is also more rapid and involves the substitution of 3 H's, not just one, forming a precipitate of 2,4,6-tribromophenol:
Other groups which are electron releasing will also "activate" the benzene ring this way by increasing electron density. They include the alkyl groups (e.g. methyl). Therefore, if you make methylbenzene (for example) by reacting chloromethane with benzene in the presence of an AlCl3 catalyst, the product will then go on to undergo further reactions much more easily without a catalyst.
Related discussions on The Student Room
- Grade boundaries »
- Edexcel A-Level Chem Paper 2 Advanced Organic and Physical Chemistry [Exam Chat] »
- Grade Growth Chronicles | From C's to A's (23-24) »
- A level chemistry question »
- Alevel Chemistry Aromatic Compounds »
- Spectroscopy help »
- Connectivity chem »
- Chemistry UV-vis spectrophotometer problem »
- Chemistry - Benzene »
- ocr a level chemistry »
Comments
No comments have yet been made