Topic 17: Chirality, Carbonyls and Carboxylic acids
For new spec (2015) Edexcel A Level. This is topic 17 on the specification, which is topic 17.1 17.2 and 17.3 in the textbook.
- Chemistry
- PolymersFunctional GroupsReactionsAldehydes and KetonesCarboxylic AcidsEstersIntermolecular forces
- A2/A-level
- Edexcel
- Created by: LouiseG
- Created on: 25-02-17 11:22
Chirality
There are two broad groups for isomerism - structural and stereoisomerism. Structural isomers have the same molecular formula but different structural/displayed formula, whereas stereoisomers share both of these; yet still differ from each other due to the arrangement of atoms in space.
In Year 1 geometric isomerism was introduced as a form of stereoisomerism, which refers to the arrangement of atoms around the C=C bond. Another form of stereoisomerism is optical isomerism.
Molecules have optical isomers when they possess chirality - the ability to have two non-superimposable forms of a molecule.
A classic example is our left and right hands; although they both contain 4 fingers and a thumb (the same "atoms" in a molecule) they are non-superimposable - you cannot place your left hand on top of your right hand, facing in the same direction, and match them up.
For a molecule to be chiral, and exhibit optical isomerism, it needs to have a chiral centre - a carbon atom bonded to four different groups.
Mirror Images
Non-superimposable atoms are the mirror images of each other. If you could rotate the mirror images above, you still could not get the atoms to all be pointing in the same direction as the other form - two atoms would always be on the opposite side of where they "should" be. You can see also that there need to be 4 different groups bonded to the central carbon (shown on the left in grey) as otherwise there would be an arrangement where the atoms lined up. In chemical formula, the carbon which is chiral is sometimes shown with a star (C*) next to it, to indicate it is the chiral centre of the molecule.
The two forms of the molecule (the optical isomers) are known as enantiomers.
Optical Activity
So why are they called "optical" isomers? This is due to the effect these isomers have on plane-polarised light; light that only oscillates in one direction only. When exposed to polarised light, each optical isomer will cause the plane of polarisation (i.e., the direction in which the light is vibrating) to rotate. This rotation is measured (as an angle from the vertical) with a polarimeter. The two forms of the isomer (the enantiomers) are labelled d- and l- ; or sometimes + and - . This refers to the direction that they rotate the plane of polarisation in. The two isomers will rotate light by exactly the same amount but in different directions, i.e. clockwise or anticlockwise.
The + or d- isomer stands for dextrorotatory, and rotates light clockwise. 'Dext' means 'right', and you can imagine, starting from the 12 o'clock position in a circle, that you move right to turn clockwise. The l - isomer stands for levorotatory, and rotates light anticlockwise. 'Levo' means left and can be explained by the same reasoning.
The diagram shows vertically polarised light being rotated by angle alpha clockwise. Make sure you know the difference between polarised (light which vibrates in one plane), a polariser (a substance that converts monochromatic unpolarised light to polarised light) and a polarimeter (the machine used to measure the optical activity of an isomer).
Racemic Mixtures
A racemic mixture is one which contains equal amounts of each optical isomer. It has no optical activity because the effect of the two isomers cancel each other out. Racemic mixtures are often found in nature, however, sometimes it is important for certain biological processes that only one isomer is present. Note it is impossible to tell which isomer is d- or l- just by looking at its formula.
Some reaction mechanisms will produce racemic mixtures whereas others will not. Measuring the optical activity of the product and reactants can be used to deduce the reaction mechanism. This can be shown through SN1 and SN2 reactions, for example, the hydrolysis of haloalkanes (Topic 16 and topic 6).
SN1 mechanism
You will know from Topic 16 that tertiary haloalkanes undergo hydrolysis through the SN1 mechanism. This is when the haloalkane first self-ionises to form a carbocation, and then the nucleophile attacks.
However, when represented in 3D, it can be seen that there are two possible isomers produced, depending on where the nucleophile attacks. Both positions are equally likely, and therefore the product is a racemic mixture of both isomers. So, if the reactant had optical activity, it will disappear in the product. This proves the SN1 mechanism occurred.
SN2 mechanism
The SN2 mechanism occurs in primary haloalkanes, and is where the nucleophile attacks the haloalkane simultaneously to the halogen bond breaking. The product will always be inverted as the nucleophile attacks on the opposite side of the molecule to the halogen, therefore the product will have the opposite effect on plane polarised light to the reactant. So if the reactant as an + isomer, the product will be the - isomer, and vice versa.
The product is the mirror image of the reactant, where the Cl group has been replaced by the OH. Note the middle "step" is a transition state, not an intermediate, and shows the instant when the OH group replaces the Cl.
Carbonyls
Carbonyls have the carbonyl functional group, C=O. Although several compounds contain this group, like carboxylic acids, only two homologous series are known as carbonyl compounds: aldehydes and ketones.
Aldehydes and ketones are functional group isomers of each other, yet exhibit different characteristics due to the position of their C=O bond. In an aldehyde, it is found at the end of a molecule, where the C=O is bonded to at least one hydrogen. In a ketone, the C=O is found within the chain, and the C in the C=O bond is bonded to two other alkyl or phenyl groups.
Physical Properties - Hydrogen Bonds
Aldehydes and ketones cannot form hydrogen bonds with themselves. This is as their electronegative O is bonded to a C, not an H. Therefore they have lower boiling points than equivalent alcohols or carboxylic acids, which have the -OH group. Aldehydes and ketones themselves have very similar boiling points. Their boiling point increases with longer chains due to the increase in London forces.
However, both compounds can form hydrogen bonds with water. They can use their :O (electron pair donor) and water can use its H d+. This means that aldehydes and ketones are soluble, however their solubility decreases as their carbon chain increases due to the interference this has with the hydrogen bonding.
Reactions of Ketones and Aldehydes - Tests
The foundation for most of the tests for ketones and aldehydes is that aldehydes can be easily oxidised, and ketones cannot. These tests can be used to confirm the presence of an aldehyde, and if the result is negative and the substance is known to be a carbonyl; a ketone.
Potassium Dichromate test
If an aldehyde is heated with acidified potassium dichromate under reflux, the solution will turn from orange to green. This is due to the Cr2O7 2- being reduced to Cr 3+ , as the aldehyde is oxidised to a carboxylic acid. With ketones, no change is seen as they cannot be oxidised.
Tollen's Reagent
If Tollen's reagent (a solution containing silver, ammonia and hydroxide ions) is added to an aldehyde and heated gently, a silver mirror forms. This is due to the Ag+ ions being reduced to Ag(s), and of course the oxidation of an aldehyde to a carboxylic acid. There is no result with a ketone.
Fehling's or Benedict's Solution
Both these reagents see the same change with an aldehyde, a blue solution turns to a brick-red precipitate when heated. This is due to the formation of a copper(I) oxide precipitate. Again, a ketone would keep the solution blue.
Oxidation and Reduction of Ketones and Aldehydes
To represent the oxidation of the aldehyde in the reactions on the previous card, [O] can be used to represent the complex role of the oxidising agent. In all examples, this reaction occurs (using Butanal as an example):
CH3CH2CH2CHO + [O] ---> CH3CH2CH2COOH
A similar notation can be used to represent the "reverse" of this, reduction. The reducing agent is Lithium Aluminium Hydride, LiAlH4. It is represented by [H]. You will remember that reduction, as well as the loss of oxygen or gain of electrons, is also the gain of hydrogen, so this makes sense. The conditions of the reaction are in dry ether, which eliminates water which may interfere with the reaction. For example:
CH3CH2CH2COOH + 4[H] --> CH3CH2CH2CH2OH + H2O
You can see this is like the reverse of oxidation, except the reaction cannot stop at an aldehyde but instead continues to a primary alcohol. Aldehydes and ketones can be reacted in the same way, for example:
CH3COCH3 + 2[H] --> CH3CH(OH)CH3
(Propanone ---> Propan-2-ol. Note not all 4 H's of the LiAlH4 need to be shown)
Nucleophilic Addition
Whilst you have met both nucleophilic substitution and electrophilic addition, you will not have come across nucleophilic addition yet (by name). Carbonyl compounds can undergo this kind of reaction due to their polar double bond:
The oxygen is more electronegative and therefore leaves the carbon in the double bond electron deficient. Compare this to the C=C bond in alkenes, where the pi bond formed had an equal density above and below, across the whole of the bond.
This means that the C d+ can be attacked by a nucleophile, which then will be added to the molecule as the pi bond in the double bond breaks.
Nucleophilic Addition of HCN in presence of KCN
You need to know the mechanism of the formation of a hydroxynitrile, when the nucleophile :CN- (from an HCN, in the presence of KCN) attacks the C d+ in the C=O bond. The CN- forms a dative bond with the carbon, which transfers its electron to oxygen to make a single bond. The oxygen, now with a (full) negative charge, attacks an H from another HCN molecule to form an organic compound with both a nitrile and a hydroxy group. The nitrile is the suffix and hydroxy the prefix, e.g. 2-hydroxypropanenitrile would be the above compound if one group attached is a CH3 and the other an H. Remember that the CN group extends the carbon chain by one, so the name stem will be different from the reactant, and that carbon number 1 is always the C in the CN group.
Evidence From Optical Activity
As with the hydrolysis of haloalkanes, you can use evidence from optical activity to support this mechanism. The product will have no optical activity as a racemic mixture forms - there is a 50/50 chance, depending on where the nucleophile attacks, of making either enantiomer. This is as the shape around the C=O bond is planar, so it is equally likely to be attacked from both above and below the molecule. Note the whole intermediate itself isn't planar.
Brady's Reagent
Brady's Reagent (2.4-DNPH , or 2,4-dinitrophenylhydrazine) is a test for carbonyl compounds. It is therefore positive for both an aldehyde and a ketone. It is not positive for other carbonyl-containing compounds, like carboxylic acids or esters.
Brady's reagent is an orange explosive solid which can be made into an orange-yellow solution. When an aldehyde or ketone is added, the positive result is the formation of a dark orange precipitate. This precipitate is a derivative of 2,4-DNPH, and can be used to identify the original carbonyl compound.
The solid is filtered, purified and dried (or recrystallised), and its melting point measured. This can be compared to a reference date book which will identify the carbonyl. This is useful, as carbonyl compounds with the same or similar melting point will have different melting points of their derivatives, so this test can be used to distinguish between them.
Iodoform / Triiodomethane Reaction
The Iodoform, or Triiodomethane (CHI3) reaction, can be used to identify methyl carbonyl groups - that is, compounds which have a methyl group attached directly to the C=O group.
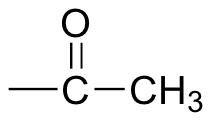
When iodine is added, in the presence of a NaOH, to a methyl carbonyl containing compound, and heated, a pale yellow precipitate forms.This is CHI3, triiodomethane. Another indication of a positive result is an antiseptic smell is produced.
Therefore the result will be positive with ethanal, ketones where the C=O is on the second carbon (-2-ones), but also alcohols that can be oxidised to either of these. This is as the conditions are oxidising, so alcohols such as ethanol will also give a positive result. Other alcohols include secondary alcohols which are -2-ols.
Carboxylic Acids
Carboxylic acids have the functional group -COOH; a carbonyl group bonded to a hydroxy group:
They are generally described as weak acids, and you will have seen them before in Topic 12 in Ka and buffers and Topic 6, as the products of oxidation of primary alcohols.
Carboxylic acids, like alcohols, can form hydrogen bonds, so have relatively high boiling points.They can use the lone pair on the either oxygen and the H bonded to the O (an electron deficient H) to form bonds. The fact they form two hydrogen bonds like this per molecule means they form dimers (above).The boiling point increases with longer chains due to increased London forces. Carboxylic acids are also soluble, as they can form hydrogen bonds with water, however their solubility decreases with increased chain length for the same reason as aldehydes and ketones; the hydrocarbon chain interferes with the bonding.
Preparation of Carboxylic Acids
Carboxylic acids can be prepared either by the oxidation of primary alcohols and aldehydes, or by the hydrolysis of a nitrile. You will already know the first method, which is done through reflux with acidified potassium dichromate:
CH3CH2OH + 2[O] ---> CH3COOH + H2O
With the hydrolysis of a nitrile, the conditions can be acidic:
CH3CH2CN + 2H2O + 2H+ ---> CH3CH2COOH + NH4+
or alkaline:
CH3CH2CN + OH- + H2O ----> CH3CH2COO- + NH3
The product here, the propanoate ion, can be converted to the acid by adding dilute acid:
CH3CH2COO- + H+ ---> CH3CH2COOH
The inorganic produced is either ammonia or the ammonium ion, depending on the method used.
Reactions of Carboxylic Acids
Most of these you will either know or have seen as applied to other compounds such as aldehydes.
1. Reduction Like aldehydes and ketones, carboxylic acids are reduced to alcohols with Lithium Aluminium Hydride, LiAlH4 in dry ether conditions.
COOH + 4[H] ---> CH2OH + H2O
2. Acid-base Like other acids, carboxylic acids react with bases to form salts. The COO- (carboxylate) ion has a -1 charge, as the H on the OH group is donated.
NaOH + HCOOH ---> HCOONa + H2O
3.Chlorination Like alcohols, carboxylic acids are chlorinated with PCl5, phosphorous pentachloride, where a Cl replaces the -OH group. The organic product is an acyl chloride:
CH3COOH + PCl5 ---> CH3COCl + HCl + POCl3
(The inorganic products are the same as for alcohols, see Topic 6)
Esterification
Carboxylic acids also can react with alcohols in acidic conditions to form an organic compound called an ester. You may have met them before at GCSE:
CH3COOH + CH3OH ---> CH3COOCH3 + H2O
The product is methyl ethanoate. Esters have the functional group -COO- and the first part of their name comes from the alcohol, or the part attached the O, and the second part from the acid, or the part attached to the C. Note that the O in the water formed comes from the acid, not the alcohol.
Esters are often pleasant-smelling compounds and are used in flavourings.
Acyl Chlorides
Acyl chlorides are derivates of carboxylic acids, where the -OH group has been replaced by the -Cl group. They are very reactive, as their C in the COCl group is very electron deficient, being bonded to two electronegative elements:
They undergo many addition-elimination reactions. These are easy to remember as, (as far as the reactions in this course go) all involve the elimination of an HCl molecule. The Cl is donated from the acyl chloride and the H from the substance it reacts with. The species left over (i.e. after the H has ben "taken off") replaces the Cl. The organic product is then easy to work out. This will be illustrated over the next slide.
Reactions of Acyl Chlorides with...
1. Water CH3COCl + H2O ---> CH3COOH + HCl
The Cl group is replaced by the -OH, forming a carboxylic acid.
2.Alcohols CH3COCl + CH3OH ---> CH3COOCH3 + HCl
Like carboxylic acids, they can be used to make esters with alcohols. Except HCl rather than H2O is the inorganic product.
3.Ammonia CH3COCl + NH3 ---> CH3CONH2 + HCl
The product of this reaction is an amide, which has the CONH functional group (an amino group attatched directly to a carbonyl).
4.Amine CH3COCl + CH3NH2 ---> CH3CONHCH3 + HCl
This is the same kind of reaction as with ammonia, except an amide with two hydrocarbon chains forms (an N- substituted amide). An amine is an organic compound like ammonia except it has hydrocarbon groups attached to the N rather than 3 H's. You will have met them in Topic 6 as the product of the reaction of a haloalkane with ammonia.
Esters
Esters, as we have seen, are organic compounds made from acids (carboxylic or acyl chlorides) and alcohols. They are useful in perfumes and flavourings, and if made in a certain way can be made into polyesters ( a form of condensation polymerisation).
We have already seen than esters can be made in acidic conditions from alcohols and carboxylic acids. You may have noticed that the reaction arrow was reversible, and so esters can also be hydrolysed in acidic conditions back to acids and alcohols:
CH3COOCH2CH3 + H2O <----(H+)---> CH3COOH + CH3CH2OH
This is not always useful as a 100% yield will never be achieved (in equilibrium)
Alkaline Hydrolysis
The reaction on the previous page isn't so useful if you want to make the products, as it will never go to completion. It is better to use alkaline hydrolysis, which goes to completion:
CH3COOCH3 + NaOH ---> CH3COO-Na+ + CH3COH
The salt can then be converted back to the carboxylic acid by addition of some H+ ions:
CH3COO- + H+ ---> CH3COOH
Polyesters
Polyesters are a form of polymer with the ester functional group. They are condensation polymers, so called because in their production a small molecule, often water, is produced. You can see this is not the same as addition polymerisation, which has 100% atom economy. Condensation polymerisation also differs in that it usually needs two monomers to occur, and each needs two functional groups. Here, we will use a diol and dicarboxylic acid.
This is as a functional group is needed on each end for the reaction to continue. You can see that, as with esterification, the OH from the di-acid and the H of the OH from the diol react, leaving an ester. At either end, this can continue with more acids and more alcohols. The repeat unit is shown with the O's at the end and bond lines to show the reaction continues.
Related discussions on The Student Room
- Grade Growth Chronicles | From C's to A's (23-24) »
- carbonyls reaction »
- carbonyls a level chemistry »
- A level chemistry optical isomerism MC questions »
- Edexcel A-Level Chem Paper 2 Advanced Organic and Physical Chemistry [Exam Chat] »
- AQA A-Level Chemistry Paper 2 (7405/2) - 19th June 2023 [Exam Chat] »
- Study leave »
- Hydrolysis »
- A-Level chemistry »
- Chemistry paper 2 igcse edexcel »
Comments
No comments have yet been made