All substances are made from atoms. Each atom is made of a nucleus - containing protons and neutrons - surrounded by electrons.
The atomic number is the number of protons in an atom. The elements are arranged in the periodic table in ascending order of atomic number.
The mass number of an atom is the total of protons plus neutrons. Atoms of the same element with different numbers of neutrons (and hence different mass numbers) are called isotopes of that element.
Atomic structure
All material things are made from atoms. There are just over one hundred different types of atom, called elements. Atoms can join together in millions of different combinations to make all the substances on Earth and beyond.
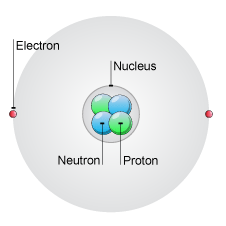
Structure of the atom
Every atom is made of a nucleus consisting of protons and neutrons. The nucleus is surrounded by electrons. Protons and electrons are oppositely charged. Neutrons have no charge. This means the nucleus of an atom is always positively charged.
An atom has a neutral overall charge because it has the same number of electrons as protons.
Protons and neutrons have the same mass. Electrons have such a small mass that this can usually be taken as zero.
Comparing the charge and mass of electrons, protons and neutrons
ProtonNeutronElectron Charge +1 0 -1 Mass 1 1 0.0005 (almost zero)
The atomic number (also called the proton number) is the number of protons in an atom.
The mass number (also called the nucleon number) is the total number of protons and neutrons in an atom.
The elements are arranged in the periodic table in ascending order of atomic number so it's easy to find the name or symbol for an atom if you know the atomic number.

Comments
No comments have yet been made