Conditions: catalyst (Fe/FeBr3 for bromination, AlCl3 for chlorination), heat.
1) Br-Br + FeBr3 --> Br+ + (FeBr4)- (for bromination)
Cl-Cl+ AlCl3 --> Cl+ + (AlCl4)- (for chlorination
2)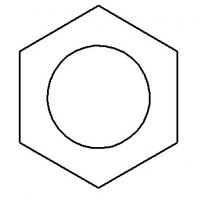 + Br+ -->
+ Br+ --> + H+ (bromination)
+ H+ (bromination)
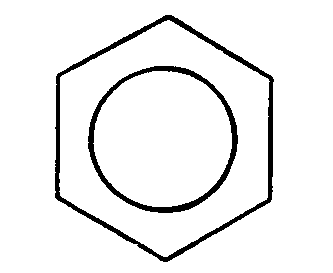 + Cl+ -->
+ Cl+ -->  + H+ (chlorination)
+ H+ (chlorination)
3) H+ + (FeBr4)- --> FeBr3 + HBr
H+ + (AlCl4)- --> AlCl3 + HCl
Comments
No comments have yet been made