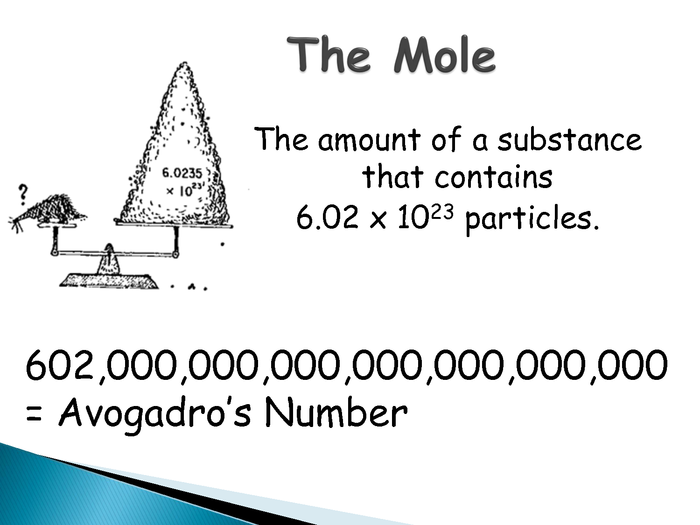
The mole is a unit of measurement-in chemistry we use it to to tell us how many atoms/molecules there are
A mole of substance=amount of substance that has same no. of particles as there are in 12g of Carbon-12.
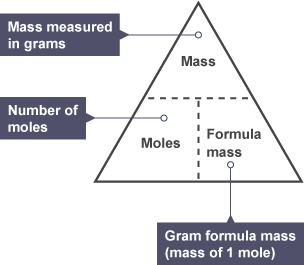 Formula mass=Relative Formula mass=Mr/Ar
Formula mass=Relative Formula mass=Mr/Ar
Comments
No comments have yet been made