Physical chem
0.0 / 5
- Created by: michaaaaaaaaaaar
- Created on: 13-04-18 11:57
enthaply notation
- Enthalpy change is the heat energy transferred in a reaction at constant pressure. the units are kjmol-1 and you write delta H to show that the measurements were made under standard conditions, and elements in their standard states.
- Standard conditions are 100 kPa, 298K, 25 degreese.
- The actual enthalpy of a system cant be measured directly but that doesnt matter because only the enthalpy change that matters.
- Enthalpy change can be measured by experiment or data books, ones in data books are usually in standard conditions which is important because changes in enthalpy are affected by temperature and pressure.
- using standard conditions means that everyone can know exactly what the enthalpy is describing.
1 of 29
types of enthalpy change
- Standard enthalpy change of reaction: enthalpy change when a reaction occurs in the molar quantities shown in the chemical equation, under standard conditions with all reactants and products in their standard states.
- Standard enthalpy of formation: enthalpy change when 1 mole of a compound is formed from its elements in their standard states under standard conditions.
- Standard enthalpy of combustion: enthalpy change when 1 mole of substance is completely burned in oxygen under standards conditions with all reactants in standard states.
- Standard enthalpy of neutralisation: enthalpy change when solutions of an acid and and alkali react together to form 1 mole of water under standard conditions.
2 of 29
exothermic and endothermic reactions
- Endothermic reactions take in energy from surroundings, this means that products of the reaction have more energy than reactants. the enthalpy change is always positive.
- example: thermal decomposition of calcium carbonate is endothermic and the enthalpy change is delta positive, photosynthesis is also endothermic as sunlight supplies energy.
- Exothermic reactions give out energy to surroundings and products have less energy than reactants so the enthalpy change is delta negative.
- example: oxidation is usually exothermic eg combustion of methane.
3 of 29
enthalpy profile diagrams
- Show you how the enthalpy changes during a reaction.
- A substance is more stable when all of its internal energy is lost so lower places in the diagram are more stable that higher ones.
- The activation energy, minimum amount of energy needed to begin breaking reactant bonds and start a chemical reaction, is the difference between the highest point and the energy of reactants.
- In exothermic reactions products are more stable than reactants as they have a lower place on the diagram.
- In endothermic reactions products are more stable than reactants as they have a lower place on the diagram.
4 of 29
Bond enthalpies
- The amount of energy per mole is called the bond dissociation energy, they involve bond breaking in gaseous compounds which makes comparisons between different bond dissociation enthalpies fair.
- Reactant bonds are broken so product bonds are formed. Stronger bonds take up more energy to break. Energy is released when bonds are formed so the reaction is exothermic. Breaking a bond is endothermic.
- If more energy is needed to break bonds than is released the reaction is delta positive, other way around is delta negative.
- Average bond enthalpies are usually used in calculations as the energy required can change depending on where it is.
- When you look up average bond enthalpies you get the energy needed to break one mole of bonds in the gas phase averaged out over may different compounds.
5 of 29
calculating enthalpy changes
- Enthalpy change of reaction = total energy absorbed - total energy released
- Calculate total energy needed to break the bonds in the reactants. This gives you total energy absorbed in the reaction.
- Calculate total energy needed to form all the new bonds in the products using the average bond enthalpies.
- Subtract absorbed energy by released energy.
6 of 29
measuring enthalpy in the lab
- Find out the number of moles of whats reacting and the temperature change.
- The way the experiment is done varies, some are done in solution where you can put a thermometer in and find temperature change using an insulated container so heat isnt gained or lost by the sides, e.g neutralisation and displacement reactions.
- Combustion reactions are harder to measure as theyre done in air. A copper calorimeter is often used containing a known mass of water is often used. The known mass is burned and the temperature change of water is measured. The heat given out should be absorbed by the water but heat can be lost to apparatus and surroundings.
7 of 29
using the equation for enthalpy change

8 of 29
calculating standard enthalpy of reaction
- 1) Calculate heat lost or gained during combustion using q=mc∆t.
- 2) Change units from J to KJ.
- 3) Calculate the number of moles of one of the reactants that caused the enthalpy change, from the mass of it that reacted.
- 4) Use the q in the reaction and divide it by moles just found, then multiply it by the moles of it used in the whole reaction. q/n (x number of moles reacting in the balanced equation).
9 of 29
calculating standard enthalpy of combustion
- 1) Calculate the amount of heat lost or gained during combustion using the equation q=mc∆t.
- 2) Change the q into KJ.
- 3) Calculate the number of molesof fuel that caused the enthalpy change from the mass reacted.
- 4) Calculate the standard enthalpy of combustion using the actual heat change for the reaction divided by moles of fuel burned, q/n.
10 of 29
Hess law
- The total enthalpy change of a reaction is always the same, no matter which route is taken.
- Useful for working out enthalpy changes you cant do by experiment.
- The law says that the energy for route 1 is the same as route 2, so if you can work out route 2 you can calculate the enthalpy for route 1.
11 of 29
using enthalpies of formation
- The enthalpy change of formation for all reactants and products that are compounds must be known.The value of enthalpy change of formation for elements is 0.
- Write under the reaction a list of all the elements present in the reaction balanced in their correct molar quantities.
- Draw and label arrows to show the enthalpy change is going from the elements to reactants and products.Label the enthalpy changes route 1 and 2, route 2 should be the direct route from elements to products.
- Multiply elements by moles in balanced equation and enthalpy change. Route 1= Route 2, plug numbers from enthalpy changes into equation.
- Subtract route 2 from route 1 and the number is the answer. The number is typically negative.
12 of 29
using enthalpies of combustion
- Write out the balanced equation.
- Add the combustion products to the diagram and make sure theyre balanced.
- Chose which reactions will form which route and label each route route 1 and 2, route 2 from reactants to combustion products and route 1 from reactants to products to combustion products.
- Subtract route 2 from route 1 and the number should be negative.
13 of 29
collision theory and activation energy
- Particles are always moving and colliding with eachother and only react when the conditions are right.
- Collision theory says a reaction wont take place between two particles unless they collide in the right direction and collide with at least a minimum amount of KE.
- Reactions that have a low activation energy happen easily, ones with high activation energies dont.
- Particles get the extra energy by heating them.
14 of 29
enthalpy profile diagrams
- Used to work out enthalpy change of a reaction and wether its endothermic or exothermic.
- If products have a lower enthalpy than reactants the reaction is exothermic.
- If products have a higher enthalpy than reactants the reaction is endothermic.
- To work out enthalpy change subtract products by reactants.
15 of 29
Boltzman distributions
- A theoretical model that has been developed to explain scientific observations.
- Its a graph that shows the number of molecules in a substance with different kinetic energies.
- This is because particles have different energies, some have a lot of energy so move fast others have little but most have a moderate speed.
16 of 29
effect of temperature on reaction rate
- When the temperature is increased molecules gain energy so move faster. This means more molecules will have at least the activation energy.
- This changes the shape on the Boltzman distribution curve and pushes the graph to the side and there is a higher curve past the activation energy, and before the activation energy the curve is lower.
- Because the molecules move faster they collide more often which is why increase in temperature increases reaction rate.
17 of 29
concentration effect on reaction rate
- If the concentration is increased the particles will be closer together so will collide more often. This increases the rate of reaction.
- Increasing the concentration means that there are more molecules in total to collide in a given volume there will be more molecules with energies above the activation energy.
- If reactants are gasses increasing the pressure will increase rate of reaction in the same way. Top line is higher concentration, bottom line is original concentration.
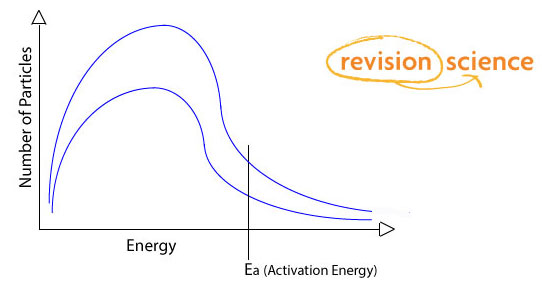
18 of 29
catalysts and how they work
- Something that makes chemical reactions happen faster by increasing the rate of reaction by providing an alternative reaction pathway with a lower activation energy.
- Theyre chemically unchanged at the end of a reaction. And are remade at the end of a reaction.
- Haber process uses an iron catalyst to increase rate of forming ammonia from nitrogen and hydrogen.
- When a catalyst is present in a reaction the reactant molecule binds to it which makes it easier to break bonds and decreases the activation energy. The broken reactant molecules then from product molecules and break away from catalyst.
- With a catalyst present the reactants still have the same amount of enegry so Boltzman distribution curve is unchanged but the line for the activation energy moves forwards.
19 of 29
homogeneous and heterogeneous catalysts
- Heterogeneous catalyst: One in a different phase from reactants, eg in Haber process the iron catalyst is a solid and the reactants are gaseous.
- Increasing the surface area of the catalyst increases the number of molecules that can react with it at the same time and increases the rate of reaction.
- Homogeneous catalyst: Catalyst in same phase as reactants. It is usually aqueous. They work by forming an intermediate species. One or more reactants combine with the catalyst and make an intermediate species which then reacts to form the products and reforms the catalyst.
20 of 29
catalysts in industry and environment
- Lots of industries rely on catalysts as they can dramatically lower production costs and help make better products.
- Using a catalyst means lower temperatures and pressures can be used which saves energy and reduces the amount of CO2 released, fossil fuel reserves are also preserved.
- Catalytic converters on cars are made from alloys of platinum, palladium and rhodium. They reduce the pollution released into the atmosphere by speeding up the reaction.
- Catalysts dont last forever and eventually need to be disposed of however they may contain toxic compounds that may leach into soil if sent directly to landfills.
- If catalysts contain valuable metals its worth recycling them, the decision whether to do this or send to landfill is made by weighing up economic and environmental factors.
21 of 29
reaction rates
- The change in the amount of reactants or products per unit time. The units are always what youre measuring.
- Rate of reaction = amount of reactant used or product formed/ time
- To measure rate of reaction you need to follow the reaction as its happening.
- To measure change in mass start a timer, make mass measurements at regualr intervals, when the mass balance stops decreasing the reaction is finished. This method is accurate and easy to use but does release gas into the room which can be dangerous or toxic so its best to do it in a fume cupboard.
- To measure gas volumes collect it in a gas syringe record how much it moves along at regualar time intervals. Its carried out in a similar way as measuring mass. It is accurate but vigorous reactions can blow the plunger out of the syringe, because no gas escapes it can be used for toxic or flammable gases.
- To work out reaction rates draw a tangent and divide Y by X where Y is the mass and X is the time.
22 of 29
dynamic equilibrium
- At equilibirum the concentrations of reactants and products stay constant. A dynamic equilibrium can only happen in a closed system so nothing can escape.
- As reactants get ised the forwards reaction slows down and the reverse reaction speeds up, after a while the forward reaction wil be going at exactly the same speed as the backwards reaction so the concentration of reactants and products wont be changing any more.
23 of 29
Le Chateliers principle
- If theres a change in concentration, pressure or temperature, the equilibirum will move to help counteract the change.
- If the pressure, concentration or temperature of a reversible reaction is changed the position of the equilibrium is altered, which means that you end up with different amounts of reactants and products at the equilibrium.
- If the position of the equilibrium moves to the left the backwards reaction is faster so you get more reactants, if it moves to the right the forwards reaction is faster so you get more products.
- eg, if you raise the temperature the equilibrium will shift to cool things down. Catalysts have no effect on the position of the equilibrium.
24 of 29
using Le Chateliers principle
- Changing concentration: If you increase the concentration of a reactant the equilibrium tries to get rid of the the extra reactant and does this by making more product. So the equilibrium shifts to the right, if you increase concentration of product the opposite happens.
- Changing pressure: Changing pressure only affects gasses. Equilibrium gets shifted to the side with fewer gas particles which reduces pressure and vise versa.
- Changing temperature: When heat energy is added the equilibrium shifts to the endothermic side to cool the reaction down and absorb the energy and vise versa. If the forward reaction is endothermic the backwards reaction would be endothermic and vise versa.
- Adding a catalyst: Catalysts have no affect as they speed up the forward and backwards reaction by the same amount, they dont increase yield but the equilibrium is reached faster.
25 of 29
ethanol production
- Produced via a reversable exothermic reaction between ethanol and steam.
- Conditions are:
- pressure of about 60-70 atmospheres
- temperature of about 300 degrees
- phosphoric acid catalyst
- Because the reaction is exothermic lower temperatures favour the forwards reaction so at lower temperatures more ethane and steam is converted to ethanol and theres a higher yield.
- Higher pressures favour the forwards reaction so the high pressures move the reaction to the side with fewer molecules of gas. Increasing pressure also increases the rate of reaction.
- Having very high pressures is expensive and need strong pipes and containers to withstand the high pressure, increasing pressure can also cause side reactions to occur, so 60-70 atmospheres is a compromise between maximum yield and costs.
26 of 29
investigating equilibrium position
- Changing temperature: In a closed system an increase in temperature will push the equilibrium in the endothermic direction and vise versa. eg NO2 (brown) can be made into N2O4 (colourless), the forward reaction is exothermic and the backwards reaction is endothermic.
- To carry out the experiment place one in an ice bath and one in a warm water bath and observe colours, the tube in warmer water will turn into a darker brown colour and the other one will go paler as the equilibrium shifts to the right to replace heat lost by favouring the exothermic reaction.
- Changing concentration: In a closed system, changing the concentrations of reactants or products will change the equilibrium position, more products moves it to the left, more reactants to the right.
27 of 29
equilibrium constant
- When you have a homogeneous reaction thats reached a dynamic equilibrium you can work out the equilibrium constant.
- It gives you an idea of how far to the right or left the equilibrium is, before you calculate Kc you have to write out the expression for it.
28 of 29
calculating equilibrium constant
- Put equilibrium concentrations into the equation.
- A change in temperature can change the position of equilibrium so Kc varies with temperature.
- Kc will increase when quantities of the products increase and reactants decrease, so an increase in Kc means equilibrium has shifted to the right and if it decreases it means the quantities of reactants have increased and products decrease and equilibrium has shifted to the left.
- The greater the increase or decrease in Kc the more the equilibrium has moved.
29 of 29
Related discussions on The Student Room
- chemistry as level equilibria help »
- Chemical engineering or pharmacy »
- Ocr a level chem »
- What affects Kp? »
- Hard friction question »
- Grade Growth Chronicles | From C's to A's (23-24) »
- Biology and chemistry »
- Chemistry vs chemical engineering »
- A level options »
- does anyone do this a level combo? »
Similar Chemistry resources:
3.0 / 5 based on 1 rating
2.0 / 5 based on 3 ratings
3.0 / 5 based on 1 rating
4.0 / 5 based on 1 rating
3.0 / 5 based on 6 ratings
4.0 / 5 based on 5 ratings
0.0 / 5
3.5 / 5 based on 5 ratings
0.0 / 5
4.5 / 5 based on 14 ratings
Comments
No comments have yet been made