Organic Analysis
- Created by: 12rmccarthy
- Created on: 15-03-18 11:39
Test tube reactions
Straightforward tests:
- Acidic?- perhaps carboxylic acid
- Solid?- suggests long C chain or ionic bonding
- Liquid?- suggests medium length C chain, polar or H bonding
- Gas?- suggests short C chain, little/no polarity
- Dissolve in water?- suggests polar groups present
Test for Alkene:
- shake with bromine water
- turns from orange to colourless
- form of electrophilic addition
Test tube reactions
Test for halogenoalkane:
- add warm aqueous NaOH so nucleophilic substitution can occur
- forms halide ions
- then acidify with HNO3 to prevent unreacted OH- ions from reacting with the silver to give a false precipitate
- add AgNO3 to form a precip of AgX.
- White- Cl
- Off white/ cream- Br
- Yellow- I
- Further testing:
Cl- dissolves in dilute ammonia
Br- doesnt dissolve in dilute but dissolves in con
I- doesnt dissolve in any
Test tube reactions
Test for alcohols:
- add potassium dichromate (K2Cr2O7) acidified with H2SO4
- orange dicrhomate ions (+6) reduced to green chromium ions (+3) Cr2O7^2- ----> Cr^3+
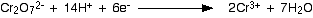
- primary oxidised to either aldehyde or carboxylic acid
- secondary oxidised to ketone
Test for aldehydes:
- warm with tollens reagent (ammonical silver nitrate) (ammonia and silver nitrate)
- silver is reduced- form a silver mirror
- Or warm with Fehlings solution- copper is oxidised to copper oxide
- blue colour turns to red precipitate
Test tube reactions
Test for carboxylic acids:
- add NaHCO3(aq)- sodium hydrogen carbonate solution
- CO2 is given off
- which is then tested with limewater
Test tube reactions
Test for carboxylic acids:
- add NaHCO3(aq)- sodium hydrogen carbonate solution
- CO2 is given off
- which is then tested with limewater
Mass spectrometry
-Mass spectrometry is used to measure relative atomic masses of atoms. But it can also be used to find relative molecular masses of organic compounds
- compound enters the mass spectometer in solution. It's ionised and the +ve ions are accelerated- fly towards the detector.
-Their time of flights are measured- these depend on the mass to charge ratio of the ion.
-Output is then presented as a graph of relative abundance against mass/charge ratio
Fragmentation:
- many of the ions within the molecular ion will break up because some of their bonds break when they are ionised. Therefore peaks of a lower mass are produced
Mass spectrometry
-Gas Chromotography Mass Spectometry used in forensic work and to detect drugs used by athletes.
- GC seperates mixtures using a stream of gas to carry a mixture of vapours through a tube packed with a solid. Different components emerge at different times- then fed into a mass spectometer which produces the mass spectrum of each.
High resolution mass spectometry:
-many mass spectometers can measure mass up to 4dp- therefore can distinguish between isotopes.
Infrared Spectroscopy
-used to help identify compounds
How it works:
- a pair of atoms joined by a bond is always vibrating
- stronger bonds vibrate faster (at higher freq)
- heavier atoms make the bond vibrate more slowly
- every bond has its own unique natural frequency that is in the IR region of the electromagnetic spectrum
- when you shine a beam of IR through a sample, the bonds can absorb energy from the radiation and vibrate more
- any particular bond can only absorb radiation that has the same frequency as the natural frequency of the bond. Therefore, the radiation that emerges from the sample will be missing the frequencies that correspond to the bonds in the sample.
Infrared Spectroscopy
What happens in the infrared spectrometer:
- a beam of IR containing a spread of frequencies is passed through a sample
- the radiation that emerges is missing the frequencies that correspond to the type of bonds in the sample
- a graph of intensity of the radiation (transmittance) against frequency of radiation (wavenumber in cm^-1)
The infrared spectrum:
- the dips in the graph (usually called peaks) represent particular bonds
Infrared Spectroscopy
Greenhouse effect:
-some gases in the atmosphere absorb IR which has been given off from materials on the surface of the earth
-Prevent IR from escaping from the atmosphere- without GG our planet would be a frozen ball of ice
-The IR strikes a molecule and causes the bonds to bend and vibrate. The extra KE may then be transmitted to other molecules like O2 and N2 for the general heating of the atmosphere
-Nitrogen and oxygen dont absorb IR as the vibrations would not change the dipoles of the molecules
Infrared Spectroscopy
Fingerprint region:
The area of an IR spectrum below about 1500cm-1 usually has many peaks due to complex vibrations of the whole molecule.
The shape is unique for any particular substance
can be used to identify the chemical (similar to how people can be identified by fingerprints)
IR usually just identifies functional groups.
Impurities can show up on an IR spectrum as random peaks that arent supposed to be there in the spectrum of the pure compound. Eg if the sample contains water- an O-H stretch is seen.
Related discussions on The Student Room
- Astrobiology »
- I enjoy chem practicals more than bio practicals. Is a chem degree right for me? »
- Grade Growth Chronicles | From C's to A's (23-24) »
- Is Chemistry better at A-level than at GCSE? »
- Keep Your Ion The Prize | Shyleen’s Second Year University GYG | 2023 - 2024 »
- OCR A-level chemistry practicals »
- What are the 5 concepts of business intelligence? »
- Chemistry at Uni? »
- Loss on ignition interference inorganic chemistry »
- Analyse how the legacy of previous approaches may affect current service deliver »
Comments
No comments have yet been made