Module 4 - Core organic chemistry
Spec notes on Module 4 of OCR A A Level Chemistry
Basic concepts, hydrocarbons, alcohols and haloalkanes, organic synthesis, analytical techniques (IR and MS)
2018
- Chemistry
- Basic conceptsHydrocarbonsAlcohols and haloalkanesOrganic synthesisAnalytical techniques (IR and MS)
- AS
- OCR
- Created by: emilyallen07
- Created on: 12-01-18 19:52
4.1.1 Basic concepts of organic chemistry
No. of carbons:
meth- 1, eth- 2, prop- 3, but- 4, pent- 5, hex- 6, hept- 7, oct- 8, non- 9, dec- 10
Nomenclature IUPAC rules for naming organic compounds;
- count carbons on longest carbon chain
- use functional groups as prefixes or suffixes
- other side chains are prefixes in alphabetical order, after no. carbon they are attached to
- multiplpe identicle side groups use di(2) tri(3) or tetra(4) before the name of their group.
General formula - the simplest algebraic formula of a member if a homologous series
Structural formula - written minimal detail that shows the arrangement of atoms in a molecule
Displayed formula - relative positioning of atoms and the bonds between them
Skeletal formula - the simplified organic formula, shown by removing hydrogen atoms from alkyl chains, leaving just a carbon skeleton and associated functional groups
4.1.1 Basic concepts of organic chemistry
Functional Groups
Homologous series - a series of organic compounds having the same functional group but with each successive member differing by CH2
Functional group - a group of atoms responsible for the characteristic reactions of a compound
Alkyl group - of formula CnH2n+1 (can be represented in diagrams as 'R')
Aliphatic - a compound containing carbon and hydrogen joined together in straight chains, branched chains or non-aromatic rings
Alicyclic - an aliphatic compound arranged in non-aromatic rings with or without side chains
Saturated - single carbon-carbon bonds only, no double bonds
Unsaturated - the presence of multiple carbon-carbon bonds (double or triple) and aromatic rings
4.1.1 Basic concepts of organic chemistry
Isomerism
Structural isomers - compounds with the same molecular formula but different structural formulae
Reaction Mechanisms
Covalent bond fissions
homolytic fission - each bonding atom recieving one electron from the bonded pair, forming two radicals
Heterolytic fission - one bonded atom recieving both electrons from the bonded pair and the pther recieving none
Radical - a species with an unpaired electron (dots • represent the electron in mechanisms)
Curly arrow - represents the movement of a lone pair of electrons in a mechanism (always starting from a bond, a lone pair of electrons or a negative charge)
4.1.2 Alkanes
Alkanes - saturated hydrocarbonsncontaining single C-C and C-H bonds as σ-bonds with free rotation of the σ-bonds.
Shape of Alkanes - tetrahedral with bond angle 109°. Electron pairs repel. lone pairs repel more than bonded pairs.
Boiling points of alkanes -
- longer chained alkanes have higher meliting points due to the induced dipole-dipole interactions (London forces) between the molecules.
- Longer chained alkanes have more electrons and more contact area for london forces to occur on.
- This means it requires more energy to break the higher number of London forces in longer chained alkanes giving them a higher melting point.
- Branched chains have lower melting points because they have a smaller surface contact area for London forces.
4.1.2 Alkanes
Reactions of alkanes
Low reactivity - High bond enthalpy and very low polarity of σ bonds means that the alkanes react much less readily with many reagents.
Alkanes as fuels
Complete combustion - C3H8 + 5 O2 ![]() 3 CO2 + 4 H2O produces CO2 and H2O
3 CO2 + 4 H2O produces CO2 and H2O
Incomplete combustion - 2 CH4 + 3 O2 ![]() 2 CO + 4 H2O produces CO and H2O Carbon monoxide is poisonous because it binds to haemoglobin in the bloodstream which prevents oxygen from being able to bind. Therefore, you can get oxygen deprivation.
2 CO + 4 H2O produces CO and H2O Carbon monoxide is poisonous because it binds to haemoglobin in the bloodstream which prevents oxygen from being able to bind. Therefore, you can get oxygen deprivation.
4.1.2 Alkanes
Free radical substitution.
In the presence of UV light, halogens react with alkanes to form haloalkanes. There is an intiiation, propogation and termination step. It works the same way as the breakdown of ozone except ozone is replaces by an alkane
4.1.2 Alkanes
Limitations of free radical substitution
Synthesis of a mixture of organic products -
Multiple substitutions can occur on each molecule. The best way to reduce the chance of this is to have an excess of your organic reactant. Then there is more chance of the organic reactant and not your already substituted product being substituted.
Also, the substitution can occur on any part of the chain so you may get an isomer of the product you are after. The desired product would then have to be removed from the mixture. There is no way to prevent this from happening.
4.1.3 Alkenes
Alkenes
Unsaturated hydrocarbon containing a σ bond (overlap of orbitals directly between the bonding atoms) and a π bond (sideways overlap of adjacent p-orbitals above and below the bonding C atoms) overlapping.
Shape of alkenes - Trigonal planar shape with 120˚ bond angle. Electron pairs repel. Alkenes contain three bonding regions and no lone pairs of electrons.
The π bond causes restricted rotation because it overlaps the σ bond and rotation would disrupt the π bonding system. This means that there is no rotation of the C=C double bonds due to the rigidity of the π bond.
4.1.3 Alkenes
Stereoisomerism - compounds having the same structural formula but with a different arrangement of atoms in space.
E/Z isomerism with multiple identical groups - Z isomers represent an alkene where the same groups are both found above or below the double bond. E isomers represent an alkene where the same groups are found on opposite sides of the double bond.
E/Z isomers where all groups are different - atoms with a higher atomic number have priority. if there are groups, you may have to look along the chain to find which group has priority e.g. a methyl group has 1C bonded to 3H but an ethyl group has 1C bonded to 2H and 1C which is bonded to 3H. At the atom where the two groups differ, the atomic number of that atom is used to determine the priority, once again with a higher atomic number giving higher priority so in this case it is the ethyl group.
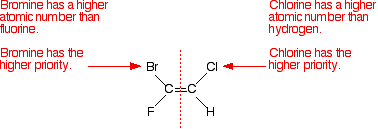
4.1.3 Alkenes
cis/trans isomerism - a special case of E/Z isomerism in which two of the substituent groups attached to each carbon atom of the C=C group are the same.
The use of E as equivalent to trans and Z as equivelant to cis is only consistantly correct when there is a H on each carbon atom of the C=C bond.
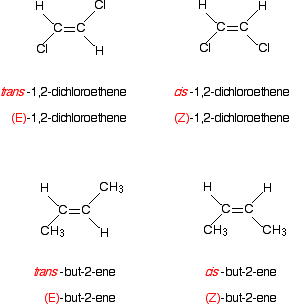
4.1.3 Alkenes
Addition reactions of alkenes
Reactivity of alkenes - alkenes are very reactive because the π bond has a relatively low bond enthalpy so it requires little energy to break. For this reason, it is very susceptible to attack from an electrophile (electron pair acceptor).
Adding H2: The hydrogen adds across the double bond to form an alkane. this reaction requires a Ni catalyst and 150°c
Producing a dihaloalkane:
/Always include the charges, dipoles and curly arrows (make sure the curly arrows are coming from the right place - either the bond or the electron/charge)/
Bromine water is the test for alkenes - it adds across the double bond and turns from orange (bromine water) to colouless (dibromoalkane).
4.1.3 Alkenes
Reactions of alkenes - steam hydration to form alcohols.
Alkenes can be hydrated by steam at 300°c and 65atm with a phosphric acid catalyst (H3PO4). The water is added across the double bond. The reaction is reversible so the yield can be low but the alkene can be recycled to increase the overall yield.
Reactions of alkenes - addition of hydrogen halides.
Alkenes undergo addition reactions of hydrogen halides to form haloalkanes. The hydrogen halides (e.g. HBr) adds across the double bond. If the alkene is unsymmetrical then there are two products formed. the amount of each product depends on how stable to carbocation formed in the intermediate step is. The stability depends on whether the carbocation is primary, secondary or tertiary (refering to how many alkyl groups surround the carbocation.). The more alkyl groups attached, the more stable meaning tertiary carbocations are the most stable and primary carbocations the least. the stability is caused by the electron donation from surrounding carbon atomstowards the positive charge.
Markownikoff's rule is that the major product from the addition of a hydrogen halide to an unsymmetrical alkene is the one where hydrogen adds to the carbon with the most hydrogens already attached to it.
4.1.3 Alkenes
Electrophilic addition mechanism:
This mechanism is relevant for every electrophilic addition reaction of alkenes (hydrogen halides, hydrogen, steam, halogens).
Always include dipoles, charges amnd curly arrows. The curly arrows represent the movement of a pair of electrons.
4.1.3 Alkenes
Polymers - the double bonds in alkenes can open up and join together to form long chains called polymers. The individual units are called monomers.
1 repeat unit looks like this:
Polymerisation is where the alkene undergoes an addition reaction with itself, forming the long chain of repeat units :
The problem with polymers is that they are so unreactive that they are not biodegradable. Therefore they are disposed of on landfill sites and by reusing them through cracking techniques, melting and remoulding them or burning them (produces toxic waste pruducts that have to be passed throuhg 'scrubbers' which neutralise them). Scientists have been developing biodegradable polymers (broken down by microorgansims) and photodegradable polymers (broken down when exposed to sunlight).
4.2.1 Alcohols
Properties of alcohols - alcohols are polar due to the electronegative hydroxyl group (-OH) which pulls the electrons away from the carbon atom in the C-OH bond.
The electronegative oxygen in a polar hydroxyl group draws electron density away from the hydrogen in the group, giving the hydrogen a slightly positive charge. This positive charge attracts lone pairs of electrons from oxygen on neighbouring molecules to form hydrogen bonds. This gives alcohols certain properties:
- when alcohols are added to water, hydrogen bonds are formed between the -OH and H2O which makes small alcohols very soluble in water. Larger alcohols are less soluble in water because more of the molecule is the non-polar chain so there is less attraction to polar H2O molecules, although they are still soluble.
- Alcohols also form hydrogen bonds with eachother due to their polarity. Hydrogen bonds are the strongest intermolecular force so alcohols have a relatively low volatility compared with similar non polar compounds such as alkanes.
4.2.1 Alcohols
Classification of alcohols
Alcohols can be classified as primary, secondary or tertiary. The classification depends on how many alkyl groups (R) the carbon which the -OH is bonded to.
Primary - C is bonded to one alkyl group
Secondary - C is bonded to two alkyl groups
Tertiary - C is bonded with three alkyl groups
4.2.1 Alcohols
Reactions of alcohols: oxidation
Oxidation by combustion of alcohols - when alcohols are burnt, they are oxidised fully to form carbon dioxide and water.
C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(g
Oxidation of alcohols by an oxidising agent (e.g. K2Cr2O7/H2SO4):
- Primary alcohols make aldehydes (distillation) and carboxylic acid (reflux)
- Secondary alcohols make ketones (reflux)
- Tertiary alcohols cannot be oxidised using an oxidising agent
4.2.1 Alcohols
Primary alcohols - forming aldehydes
To form an aldehyde, the primary alcohol must be distilled so that the aldehyde is removed as soon as it is formed and it only oxidies once.

If the primary alcohol is oxidised fully it will form a carboxylic acid. To do this the primary alcohol must be refluxed so that it oxidises twice.
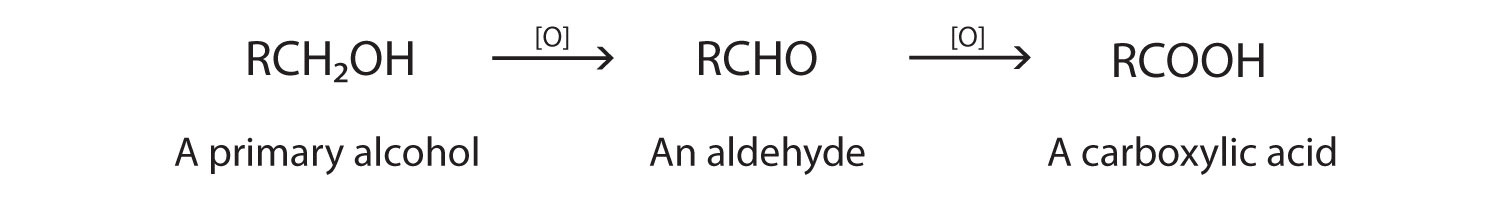
4.2.1 Alcohols
Oxidising secondary alcohols - forming ketones.
Refluxing a secondary alcohol in the presence of an oxidising agent (e.g. K2Cr2O7/H2SO4) will form a ketone.
![]()

Tertiary alcohol do not oxidise in these conditions (distillation or reflux) because there is no H bonded to the carbon for the [O] to remove.
4.2.1 Alcohols
Reactions of alcohols
Elimination reaction to form alkenes -
When mixed with a concentrated acid catalyst (e.g. H2SO4 or H3PO4) and heated, an alcohol will turn into an alkene. This is an example of dehydration.
E.g. dehydration of butan-2-ol
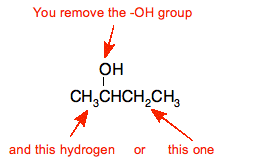
That leads to these products
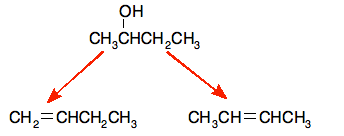 The products are but-1-ene and but-2-ene. The but-2-ene can have both an E and a Z isomer due to the stereoisomerism.
The products are but-1-ene and but-2-ene. The but-2-ene can have both an E and a Z isomer due to the stereoisomerism.
4.2.1 Alcohols
Reactions of alcohols
Substitution with halide ions to form haloalkanes.
- Alcohols react with compounds containing halide ions (such as from NaBr) in a substitution reaction.
- The hydroxyl group is replaced with the halide ion to form the haloalkane.
- The reaction can happen at room temperature but requires an acid catalyst (e.g H2SO4 or H3PO4) in order to happen.
Haloalkanes
Substitution reactions of haloaklanes -
When a haloalkane is reacted with hot aqueous alkali, a nucleophilic substitution occurs.
It must be refluxed.
Haloalkanes
To test for the rate of hydrolysis of C-halogen bonds:
1. Add water, to react with the haloalkane to form an alcohol.
2. Adding silver nitrate to the solution will show how fast the reaction occurs, a precipitate forms when the substitution has occured.
3. The faster the precipitate forms, the weaker the C-halogen bond is (the smaller the bond enthalpy).
4. Iodine forms a yellow precipitate first. Then bromine forms a cream precipitate. Next, chlorine forms a white precipitate.
C-I < C-Br < C-Cl < C-F
Haloalkanes
Using organohalogen compounds has environmental impacts:
UV radiation breaks down CFCs in the upper atmosphere, resulting in the breakdown of earths protective ozone layer.
initiation - C2F2Cl2 → C2F2Cl • + •Cl
propogation - •Cl + O3 → •Cl O + O2
•Cl O + O → •Cl + O2
Related discussions on The Student Room
- A-level chemistry. Draw a labelled diagram to show the formation of the π-bond. »
- Grade Growth Chronicles | From C's to A's (23-24) »
- !!! A level choices - Chem or Computer science »
- uni modules and how they apply to my course »
- I enjoy chem practicals more than bio practicals. Is a chem degree right for me? »
- Keep Your Ion The Prize | Shyleen’s First Year University GYG | 2022 - 2023 »
- Worried about studying Chemistry at UNI »
- Opinions »
- Undergraduate chemistry »
- Uni of Manchester pharmacy help »
Comments
No comments have yet been made