Module 2, Section 4, Enzymes
- Created by: SparklingABS
- Created on: 21-09-16 14:22
Enzymes
Enzymes:
-are biological catalysts which speed up reactions
-catalyse metabolic reactions.
-can affect structures in an orgnaism as well as functions.
-action can be intracellular or extracellular
-are globular proteins
-have an active site with a specific shape
-shape is determined by tertiary structure
-for enzyme to work, shape of active site must be complementary to the substrate.
Reducing Activation Energy
They do this in two ways:
- if two substrate molecules need to be joined, being held together by enzymes, reduces repulsion, so they bond easily.
- if enzyme is catalysing a breakdown, the active site puts strain on the bonds in the substrate making it easier to break.
Lock and Key Model
The lock and key hypothesis is where the substrate fits into the enzyme in the same way that a key fits into a lock. The substrate has to be complementary to the active site shape and only one substrate fits a certain active site.
Induced Fit Model
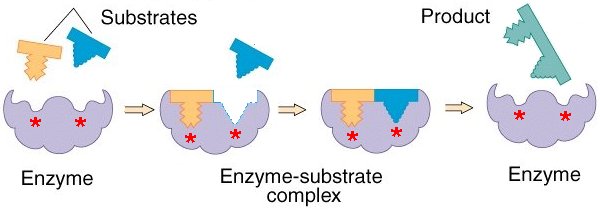
This suggests that as the substrate binds, it makes the active site change slightly to fit the substrate more closely.
The induced fit model is now more widely accepted than the lock and key model. This is an example of how a theory can change when new evidence comes along.
Temperature
As temperature increase, the molecules have more kinetic energy, therefore move faster. This makes it more likely for substrate to collide with enzyme and produce an ES complex.
However, once past optimum temperature, the enzyme denatures because bonds that hold the tertiary structure in place break and the active site changes shape.
Temperature Coefficient
Q10 - value for the reaction. It shows how much the rate of reaction changes when temperature is raised by 10°C
pH
Above and below the optimum, the H+ and OH- ions mess up the ionic and hydrogen bonds that hold the enyme's tertiary structure in place. This makes the active site change shape and the enzyme is now denatured.
Enzyme Concentration
The more enzyme molecules present, the more likely a substrate will collide with it, therefore increasing the rate of reaction.
But if substrate is limited, it comes to a point where all substrate molecules are being dealt with, so adding more enzyme will have no further effect.
THE SUBSTRATE IS NOW THE LIMITING FACTOR
Substrate Concentration
The more substrate molecules present, the more likely they will collide with an enzyme, therefore increasing the rate of reaction.
This will happen up until 'saturation' point. This is when all active sites are full, so adding more substrate will have no further effect.
THE ENZYME IS NOW THE LIMITING FACTOR
Substrate concentration decreases with time if no variables are changed. Due to this, the intial rate may be the highest rate in a given reaction.
Cofactors and Coenzymes
Cofactors = inorganic molecules/ions
- helps to bind enzymes + substrate together
- aren't used up in the reaction
- example = Cl- for amylase
Cofactors = organic molecules/ions
- called coenzymes
- participate in reaction and are changed
- act as carriers to move chemical groups between different enzymes
- continually recycled
- example = vitamins
Cofactor = tightly bound to an enzyme
- known as a prosthetic group
- permanently bound by covalent bonds
- example = Zn2+ for carbonic anhydrase
Competitive Inhibition
- Have similar shape to substrate
- Competes with substrate to bind with active site
- How much inhibition depends on concentration of inhibitor and substrate
- High concentration of substrate means a fast rate of reaction
- High concentration of inhibitor means a slow rate of reaction
Non-Competitive Inhibition
- Bind to enzyme's allosteric site
- Causes active site shape to change – substrate can no longer bind
- Increasing substrate concentration will do nothing because the enzyme will still be inhibited and have the wrong shaped active sit
Reversible and Non Reversible Inhibitors
This depends on strength of bonds:
- Strong covalent bonds = irriversible
- Weak hydrogen bonds/ionic bonds = reversible
Drugs and Metabolic Poisons
Drugs
- antiviral drugs prevent virus from replicating
- antibiotics (e.g. penicillin - weakens cell wall and causes wall to burst, bacteria killed)
Metabolic Poisons
- cyanide - irreversible inhibitor - affects respiration reactions
- Malonate - affects respiration reactions
- Arsenic - affects respiration reactions
End Product Inhibition
Metabolic pathway = a series of connected metabolic reactions with each reaction being catalysed by a different enzyme.
- Many enzymes are inhibited by the product of the reaction - the products stays in the active site until product level drops, which causes level of inhibiton to fall, so product leaves active site and enzyme can start functioning again.
Enzyme inhibition - protects cells
Enzymes are sometimes synthesised as inactive precursors in metabolic pathways.
This is done to prevent the pathways causing damage to cells.
Example - some proteases stop them from damaging cells in which the protease enzyme is made in.
Related discussions on The Student Room
- biology a level electrophoresis question »
- Chemistry paper 1 a level »
- data handling - putting figures into graphs »
- Chemistry B (Salters) 'Fndmntl Chmstry Wtn' [Exam chat] »
- BTEC applied science Unit 3 2023 »
- Grade Growth Chronicles | From C's to A's (23-24) »
- 25 mark essay question »
- Applied Science Unit 3 investigation skills »
- Applied science unit 3 »
- AQA A - level biology graphical questions »
Comments
No comments have yet been made