Chemistry unit 5
- Created by: Bree-Ffionn
- Created on: 14-09-19 10:31
Basic behaviour of metal oxides and hydroxides
- Bases are substances that accept hydrogen ions, H+
- These ions are oftern referred to as protons.
- Sometimes bases are called proton acceptors.
- Both metal oxides and metal hydroxides can behave as bases.
Metal oxides
- The oxide ions in metal oxides can accept protons and neutralise acids.
- Calcium oxide will neutralise sulfuric acid to form calcium sulfate.
- CaO(s) + H2SO4 -> CaSO4 + H2O
- O2- + 2H+ -> H2O
- Calcium oxide (lime) is used in agriculture to neutralise acidic soil.
- This is a important reaction as if the pH of soil is too low, the plant will tend to absorb toxic ions that affect root growth.
Metal hydroxides
- Metal oxides will form hydroxides when they react with water;
- MgO(s) + H2O (i) -> Mg(OH)2(s)
- Hydroxide ions can accept protons and neutralise acids.
- Magnesium hydroxides is used as an antacid to relive the symptoms of indigestion and heartburn, by neutralising hydrochloric acid that has entered the oesophagus.
- Mg(OH)2(s) + 2HCL(aq) -> MgCL2 (aq) + H2O
- OH- (aq) + H+ (aq) -> H2O
- Calcium hydroxide is used to neutralise acidic effluent in the chemical industry, like a dilute solution of sulfuric acid in the waste water from the manufacture of polymers.
- Ca(Oh) 2 + H2SO4 -> CaSO4 + 2H2O
Amphoteric oxides
- Amphoteric oxides can behave as either aids or bases.
- Elements that form such oxides are normally in the middle of a period.
- Aluminium oxides will not dissolve in water, but will react with acids to form a salt and water in a similar way to other metal oxides.
- It will react with HCL(Aq) in warm conditions;
- AL2O3 + 6HCL -> 2ALCL3 + 2H2O
- It will also react with bases to form aluminates.
- It will react with NaOH to form sodium tetrahydroxoaluminate, which is used in the Bayer process to convert the mineral bauxite into aluminiu oxide;
- AL2O3 + 2NaOH + 3H2O -> 2NaAL(OH)4
Alumina
- This is a form of aluminium oxide found in the mineral bauxite.
- It is extracted and purified using the Bayer process.
- The stages in the process are;
1) Crush the bauxite
2) React with NaOH at 170 degree celcius to form NaAL
3) Filter out solid impurities
4) Allow to crystallise to form AL(OH)3
5)Heat in rotary kiln to form AL203
- Most of the alumina separated in this way is used in the Hall-Heroult process to form aluminim by electrolysis.
- Some is used as a refractory material in kilns.
- These are materials that retain their strength and are chemically stable at high temperatures.
Extraction of aluminium
- Aluminium ore, bauxite, is mined and then processed to form alumina.
- Molten alumina is then electrolysed using the Hall-Heroult process.
- Cryolite is added to the alumina to lower the melting point and save energy.
- The lining of the steel tank is made from carbon, which acts as negative electrode.
- Aluminium ions are reduced to form molten aluminium:
AL3 + 3e- -> Al
- Molten aluminium can be drained off and cast into ingots.
- The positive electrodes are made from carbon.
- Positive electrodes are made from carbon.
- Oxide ions are oxidised to form oxygen gas;
2O2- -> O2 + 4e-
- The carbon electrodes have to be replaced regularly as they react with the oxygen as it forms.
Extraction of titanium
- The main titarium ore is rutile, which contains titanium dioxides, TiO2.
- Titanium has a similar reactivity to aluminium, it is not generally extracted by electrolysis as the titanium formed will oftern have tree like crystals, that can affect the electrodes.
- Side-reactions with titanium ions at both electrodes can lead to impurities.
- Most titanium is extracted using the Kroll process;
Titanium dioxides - coke and chlorine are heated tigether at about 900 degree celcius to form titanium chloride.
Tio2 +2C+2cl2 -> TiCL4 +2CO
Magnesium is used as a reducing agent to form titanium
TiCL4 + 2mg -> Ti + 2MgCL2
- Process is expensive due to the large amounts of energy needed to create the very high temperatures involved.
Electrolysis of brine
- A solution of NaCL produces chlorine, hydrogen and sodiun hydroxide when electrolysed.
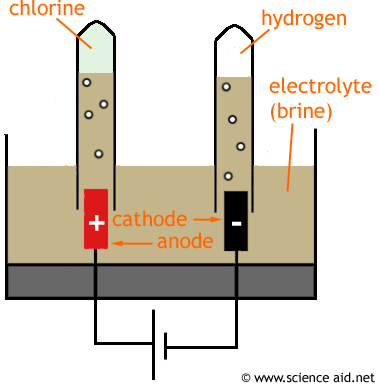
Reactions at each electrode
- Chloride ions react at the positive electrode (anode) to form chlorine gas;
2CL- -> CL2 + 2e-
- Each chloride ion has lost an electron so the reaction is oxidation.
- Hydrogen ions, from the water, react at the negative electrode to form hydrogen gas
2H+ + 2e- -> H2
- Each hydrogen ion has gained an electron so the reaction is reduction.
- The reamining sodium ions and hydroxide ions stay in solution forming sodium hydroxide solutiom, oftern referred to as caustic soda.
Diaphragm cell
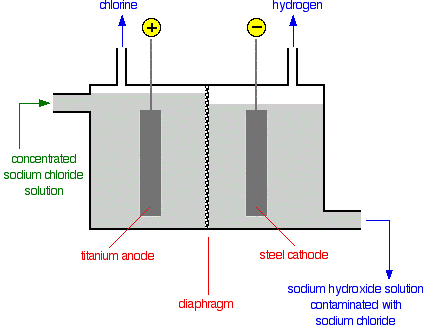
- Used in the chlor-alkali industry.
- A diaphragm that divides the cell.
- Porous, it allows brine to pass from one side to the other but prevents chlorine and hydrogen gas from passing through.
- Sodium hydroxide formed is still mixed with brine.
- Sodium chloride is less soluble than sodium hydroxide = can be recrystallised.
Membrane cells
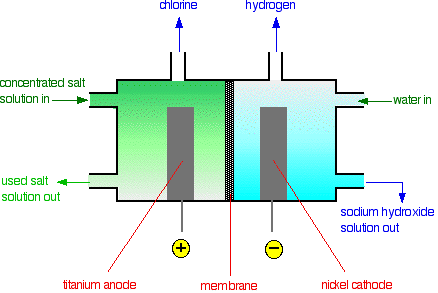
- Used in the chlor-alkali industry, has a ion-exchange membrane, divides the cell.
- Allows positive sodium ions, but will not allow negative chloride ions, to pass through the anode side to the cathode sides.
- The brine only enters the cell from the anode sode, the sodium hydroxide left over at the cathode side is not contaminated by sodium chloride.
Pros and cons of the diaphragm cell
- Cheaper to construct.
- Needs replacing regularly.
- Purity of the sodium hydroxide formed is lower and the cell uses more energy per tonne of chloride produced.
Pros and cons of the membrane cell
- More expensive to construct.
- Membrane needs little maintenance.
- Purity of the sodium hydroxide formed is high and the cell used less energy per tonne of chlorine produced.
Bonding in alkanes
- The overlap of orbitals in alkanes results in the formation of sigma bonds between carbons and hydrogens, and between adjacent carbons.
- These single bonds are free to rotate.
- Carbon orbitals involved in the bonding are called sp3 hybrid orbitals.
- Formed when the 2s orbitals and three 2p orbitals in carbon's outershell rearranged themselves into four identical orbitals, each containing one unpaired electron.
- This process is called hybridisation.
Boiling points of alkanes
- Longer carbon chains have stronger London forces as they have more elcectrons.
- Electron density of the larger e;ectron clouds fluctuates more readily, so instantaneous dipoles are stronger in magnitude.
- More energy is required to break the London forces.
Boiling points of alkenes
- Overlap of orbitals in alkenes results in the formation of sigma bonds between carbons and hydrogens, and forms one of the bonds between adjacent carbons.
- Carbon orbitals involved in the boning are called sp2 hybrid orbitals.
- They are formed when the 2s and two of the 2p orbitals in carbon's outer shell rearranged into three identical orbitals, each containing one electron.
- The remaining p orbitals in each carbon overlap sideways to form pi bond.
The pi bond
The pi bond has two significany effects
1) It restricts rotation around the double bond. This means groups attached to the carbons in the double bond are locked in position, which can lead to the formation of stereoisomeres.
2) The high electron density influeneces many of the chemical reactions of alkenes. Electron deficient species will be attracted to and oftern react with the double bond in addition reactions.
Stereoisomersism in alkenes
- Can form structural isomers.
- They are restricted about the carbon-carbon double bond in alkenes can lead to stereoisomers.
- These are compounds with the same structural formula, but their atoms have a different arrangement in space.
- The haloalkene CHCICBrCH3 has two possible spatial arrangements;
Bond length and strength in hydrocarbons
- The double carbon-carbon bonds in hydrocarbons are shorter and stronger than carbon-carbon single bonds, as the attraction between the nuclear and the shared pairs of electrons is greater.
- Not twice as strong, p orbitals overlap is less effective than overlap by hybrid orbitals.
- Hydrocarbon benzene would have long and short carbon-carbon nonds of diffrent strengths.
Reaction with halogens
- This reaction is called a free radical substituation.
- The mechanism of a reaction shows what occurs at each stage of the process.
- The stages in this mechanism are;
1) Initiation - homolytic bond fission of a halogen to form halogen free radicals. Uses UV light or heat.
2) Propagation - steps where one free radical reacts and forms a diffrent radical.
3) Termination - combination of any two free radicals to form a stable product
Key ideas about the addition reactions of alkenes
- The electron-rich c=c bonds in alkenes is susceptible to attack by electrophiles.
- Electrophiles are species that can accept a pair of electrons.
- Electrophiles are either positive ions or polar molecules with partial charges.
- Some species can become an electrophile by passing near to a C=C double bond, as its high electron density is enough to induce a dipole.
Addition reaction mechanism
- This is the series of steps showing how ethene underoes an addition reaction with bromine.
- A curly arrow shows the direction in which a pair of electrons move.
- The electrophiles or alkene will change, the basic steps of the mechanisms are the same.
- The electrophilic ends in each of the other reactants are shown in red.
- with hydrogen halides
-with sulfuric acid
- with steam
Addition to asymmertic alkenes
- Alkenes like ethene are symmetric.
- Alkenes like propene, have diffrent groups on either side of the double bond, so are asymmetric.
- When asymmetric alkenes react with asymmetric electrophiles more than one product forms;
Propene
2 - bromopropane major product
1 - bromopropane minor product.
Key ideas about the addition reaction of alkenes
- The electron-rich C=C bond in alkenes is susceptible to attack by electrophiles.
- Electrophilies are species that can accept a pair of electrons.
- Electrophiles are either positive ions or polar molecules with partial charges.
- Some species can become an electrophile by passing near to a C=C double bond, as its high electron density is enough to include a dipole.
Addition polymerization of alkenes
- Addition polymersiation involves the joing together of many alkene minomers to form a large saturated molecule called a polymer.
- During these reactions, free radicals are formed during an initation step, oftern by reaction with an oxygen or peroxide catalysts.
- During propagation steps, free radicals react with a monomer forming free radical with a longer carbon chain.
- This process sontinues forming longer and longer free radicals.
- Two long free radicals then combine to form the polymer in a termination step.
Cracking of alkanes
- In the cracking of alkabes, larger hydrocarbons with lower commercial demand are broken down by thermal decomposition into smaller, higher demand alkanes and alkenes.
- The commercial reaction can be replicated in the labatory using liquid paraffin, ehich contains decane, C10H22.
- The pieces of china act as a catalyst and the products are octane and ethene;
C10H22->C8H18 + C2H4
- An example of cracking in industry uses the fraction diseal, with hydrocarbon chains in the range 14-20 carbons.
- This is cracked using heat and a zeolite catalyst.
- The major products are ethene and propene, used to make polymers, and petrol, used in fuell.
Hydration of ethene
- Ethene, produced from the cracking of crude oil fractions, can be converted into ethanol by direct hydration with steam.
- C2H4 + H20 -> CH3CH2OH
- The reaction is exothermic, so higher temperatures produce a lower yield.
- Due to increased rate of reaction to a suitable level, the process is carried out at 500K with a phosphoric acid catalyst.
- High pressure of around 60 atm is used.
- This pressure gives a rekatively high yield, but even higher pressure would compromise safety, be expensive and cause some of the ethene to start to polymerise.
Combustion of alkanes
- The commercial use of alkanes in combustion reactions is linked to the length of the carbon chain.
- Shorter hydrocarbons can be liquified under pressure.
- Can then be stored in tanks or canisters.
- Hydrocarbons such as octane are found in the fuel petrol.
- Longer hydrocarbons are found in fuels such as diseal.
Dissolving ionic solids
- When soluble ionic solids, such as sodium chloride, dissolve in water, two processes occur.
- The ions break away from each other in the ionic lattice.
- Process requires energy, so is endothermic.
- Newly separate ions then form electrostatic interactions with the slightly charged water molecules.
- Process releases energy so is exothermic.
- Energy released in the second process compensates for the energy requirement when the lattice breaks, meaning water is a good solvent for many ionic compounds.
Related discussions on The Student Room
- BTEC Applied Science Exam Revision »
- BTEC Level 3 Applied Science Unit 1 May 2022 Exams »
- Trek To The End Of Year 13 ❀◕ ‿ ◕❀ »
- Pharmacy/a levels »
- Unit 5 applied science 2024 »
- Btec applied science- unit 7 »
- BTEC Applied Science Unit 5 2023 »
- Biology and chemistry »
- Chances of getting in St Andrews for a Biology or Chemistry degree with a HNC/D? »
- Access course for Pharmacy »
Comments
Report