Potassium manganate crystals (KMnO4) in water
Bromine (Br2) liquid is placed in a glass jar with a lid & the vapour evaporates from the volatile liquid and fills the gas jar. Then a second gas jar of air is placed upside down on top of the original bromine filled gas jar and the lid is removed. The bromine gas gets distributed evenly between the two gas jars after some time. Diffusion takes place faster in a gas than a liquid.
Cotton wool soaked in ammonia (NH3) is placed at one end of a tube and cotton wool soaked in hydrogen chloride (HCl) is placed at the other. HCl and NH3 molecules diffuse through the air towards eachother & react to form a white powder called ammonium chloride (NH4Cl) when they meet.
HCl(g) + NH3(g) 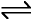 NH4Cl(s)
NH4Cl(s)
Comments
No comments have yet been made