Stars emit electromagnetic radiation between ULTRAVIOLET and INFRARED
A SPECTOGRAPH analyses frequencies of a beam of light
Absorption spectra is COLOURED with BLACK lines. this is where the frequencies are missing. Atoms in the star have absorbed the light at this frequency.
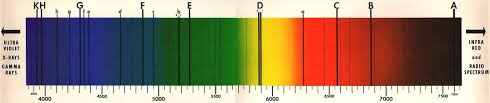
Absorption in the sun & other stars.
Different temp = different particles are able to absorb light. So even the sun is mainly H & He, they don't show because they can't absorb light at temp. of sun.
Comments
Report