Density is the mass per unit volume. It can be measured in several ways.
The most accurate way to calculate the density of any solid, liquid or gas is to divide its mass in kilograms by its volume (length × width × height) in cubic metres.
Density can be found using the equation:
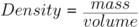
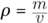
A eureka can is a container large enough to hold the object with a spout positioned near the top. The can is filled to the top with water and the object placed in it. The volume of the object is equal to the volume of the water that is forced through the spout.
Eureka cans are named after a scientist called Archimedes who first recorded this idea. They are sometimes also called displacement vessels.
Comments
No comments have yet been made