Chemistry C1 (part 2)
0.0 / 5
- Created by: lucylulou
- Created on: 15-05-16 18:40
Cracking Crude Oil
- Long-chain hydrocarbons form thick gloopy (viscous) liquids eg. tar > not that useful
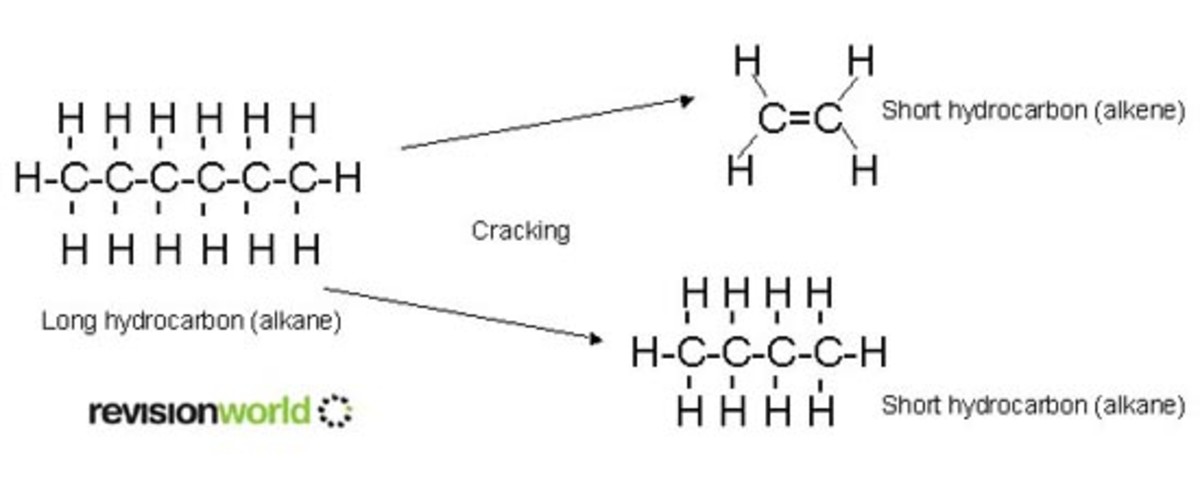
- Most longer molecules produced from fractional distillation are turned into smaller ones by the process of cracking
- Some products from cracking are useful as fuels, eg. diesel > cracked to form petrol, paraffin
- Ethene can also be produced > needed for making plastics
- Cracking is a thermal decomposition reaction (breaking molecules down with heat)
- First the long-chain hydrocarbon is heated to vaporise it > passed over a powdered catalyst (aluminium oxide) at around 400 - 700ºC
- The long-chain molecules split apart or 'crack' on the surface of the catalyst
- Most products of cracking are alkanes and alkenes.
1 of 4
Alkenes & Ethanol
- Alkenes > hydrocarbons > double bond between 2 carbon atoms in their chain C=C
- They're unsaturated because they can make more bonds - double bond can open, allows the 2 carbon atoms to bond with other atoms
- Alkenes: ethene (2 Cs), propene (3 Cs), butene (4 Cs) general formula: CnH2n
- Test for alkenes > add to bromine water > the alkene will decolourise the bromine water > orange to colourless (double bond is opened and forms bonds with bromine)
- Ethene (C2H4) can be hydrated with steam (H2O) with a catalyst > produce ethanol
- atm. it's a cheap process > ethene is fairly cheap, not much wasted. Ethene's from crude oil (non-renewable) > will run out > therefore the process will get more expensive
- Ethanol can also be produced by fermentation of sugar (renewable) + yeast
- sugar -> carbon dioxide + ethanol
- Needs a lower temp. and simpler equipment. It's cheap fuel
- Disadvantages: the ethanol produced isn't very concentrated, it has to be distilled and purified
2 of 4
Alkenes > Polymers
Alkenes > polymerisation > polymers
- Joining together lots of small alkene molecules (monomers) to form very large molecules called polymers
- Eg. join up lots of ethene molecules > produce polyethene
- Physical properties depend on: what it's made from and temperature & pressure of polymerisation
- eg. Polyamides are stronger than polyethene. Polyethene made at 200ºC with 2000 atmospheres pressure > flexible, low density. Polyethene made at 60ºC and low pressure + catalyst > rigid, dense
- Light, stretchable polymers > plastic bags. Elastic polymer fibres > LYCRA fibre > tights. Waterproof coatings for fabrics (polymers). Dental polymers > resin tooth fillings. Polymer hydrogel wound dressings > keep moist. Biodegrable packaging > polymers & cornstarch. Memory foam (smart material) > polymer gets softer when warmer.
- Most polymers arn't biodegradable > hard to get rid of > contribute to landfill or recycle
- Polymers are cheaper than metal, but as crude oil runs out > get more expensive
3 of 4
Plant Oils
Some fruits and seeds contain lots of oil > extracted for food/fuel
- Plant material crushed > pressed between metal plates > oil squeezed out
- OR use a centrifuge to separate oil from crushed plant OR use solvents
- Distillation refines oil and removes water, solvents & impurities
- Vegetable oils (used in food) have a very high energy content and contain nutrients & essential fatty acids (metabolic processes)
- Benefical:
- higher boiling point than water, cook foods at higher temp. and faster
- gives a different flavour
- get more energy from foods cooked in it
- Vegetable oils (eg. rapeseed & soybean oil) can be turned into fuels (provide lots of energy)
- Useful fuel from vegetable oils > biodiesel.
- Similar properties to ordinary diesel - burns in the same way, so it can be used to fuel a diesel engine.
4 of 4
Related discussions on The Student Room
- Energy dissipated »
- Maths for economists - constrained maximisation questoin »
- Senior Physics Challenge 2022 »
- circle question help »
- Learning a 2nd language »
- Paramedic science- time out after college »
- best way to revise maths »
- Isaac Senior Physics Challenge 2024 »
- Learning a 2nd language »
- Senior Physics Challenge Isaac Physics »
Similar Chemistry resources:
0.0 / 5
0.0 / 5
1.0 / 5 based on 1 rating
3.0 / 5 based on 1 rating
0.0 / 5
0.0 / 5
0.0 / 5
0.0 / 5
0.0 / 5
0.0 / 5
Comments
No comments have yet been made