Chemistry C1 (part 1)
- Created by: lucylulou
- Created on: 07-03-16 20:05
Atoms & Elements
<- THE ATOM
The nucleus has an overall positive charge
- Protons have a positive charge
- Neutrons have no charge
- Electrons have a negative charge > they occupy shells around the nucleus
Atoms have no charge overall (neutral)
- Charge on electrons is the same as charge on protons, but opposite
- Number of protons = number of electrons
- If electrons are ADDED or REMOVED, the atom becomes CHARGED and is then an ION
Elements consist of one type of atom only, no. of protons determines the type of atom
The Periodic Table
The Periodic Table is made up of groups and periods
Horizontal columns > PERIOD
Vertical columns > GROUPS
- Elements in the same group have the same no. of electrons in their outer shell
- Elements in the same group have similar properties (eg. group 1 elements are all metals and all react in the same way)
- Group 1 - ALKALI METALS
- Group 2 - ALKALI EARTH METALS
- Between group 2 and 3 - TRANSITION METALS
- Group 7 - HALOGENS
- Group 0 - NOBLE GASES > full outer shells > stable and unreactive
- Top number (higher no.) > MASS NUMBER... total no. of protons and neutrons
- Bottom number (lower no.) > ATOMIC NUMBER... no. of protons (same as no. of electrons)
- Mass number - atomic number = number of neutrons
Electron Shells
Electron Shell Rules
- Electrons occupy SHELLS (aka. ENERGY LEVELS)
- The LOWEST energy levels are always FILLED FIRST (one closest to nucleus)
- Only a certain number of electrons are allowed in each shell 1st shell: 2, 2nd shell: 8, 3rd shell: 8
- Atoms 'want' to gain a full outer shell (noble gases have full outer shells - they are stable)
- Most atoms have an outer shell which isn't full > this makes the atom want to react to fill it and become stable
Electronic Structures
- Use the periodic table to find out the atomic number
- This gives us the number of protons and also the number of electrons
- The 1st shell only takes 2 electrons, the 2nd can take 8 electrons
E.g. Nitrogen (N) has an atomic number of 7, it has 7 protons and 7 electrons therefore its electronic structure is '2, 5'
Compounds
Atoms Join Together to make Compounds
- When dif. elements react, atoms form chemical bonds with other atoms to form compounds. It's usually difficult to separate the 2 original elements out again
- Making bonds involves atoms Giving Away, Taking or Sharing electrons
IONIC Bonding(eg. Sodium Chloride)
- A compound is formed from a metal and a non-metal
- The metal atoms lose electrons to form positive ions and the non-metal atoms gain electrons to form negative ions
- The opposite charges of the ions mean that they're strongly atttracted to each other (electrostatic attractions)
COVALENT Bonding(eg. Water)
- A compound is formed from non-metals
- Each atom shares an electron with another atom
- Each atom has to make enough covalent bonds to fill its outer shell
Equations
A Formula shows what Atoms are in a Compound (eg. one molecule of H2SO4 , is made up of 2 hydrogen atoms, 1 sulphur atom and 4 oxygen atoms)
Atoms Aren't Lost or Made in Chemical Reactions
- There has to be the same atoms at the end of a chemical reaction as there was at the start. They're just rearranged.
- Balanced symbol equations show the atoms at the start (reactants) and the atoms at the end (products) and how they're arranged. For example...
Word equation: magnesium + oxygen > magnesium oxide
Balanced symbol equation: 2Mg + O2 > 2MgO
![]()
Atoms aren't gained or lost > the mass of the reactants must equal the mass of the products
(eg. if 6g of magnesium reacts with 4g of oxygen, you'd get 10g of mgnesium oxide)
Limestone
Limestone is Calcium Carbonate (CaCO3)
Limestone is quarried out of the ground > it is a useful building material
When it is heated it thermally decomposes to form calcium oxide and carbon dioxide
Calcium oxide reacts with water to produce calcium hydroxide. Calcium hydroxide is an alkali and so can be used to neutralise acidic soil
Calcium hydroxide can be dissolved in water (aq) > this solution is called limewater. Limewater can be used to test for carbon dioxide. If you bubble a gas through limewater, it will turn cloudy if the gas contains CO2 , this is due to the formation of calcium carbonate.
When calcium hydroxide (aq) is added to carbon dioxide, calcium carbonate and water are formed.
- Cement is made when powdered limestone is heated in a kiln with powdered clay
- Mortar is made when cement is mixed with sand & water (stick bricks together)
- Concrete is made when cement, sand & aggregate (water + gravel) is mixed
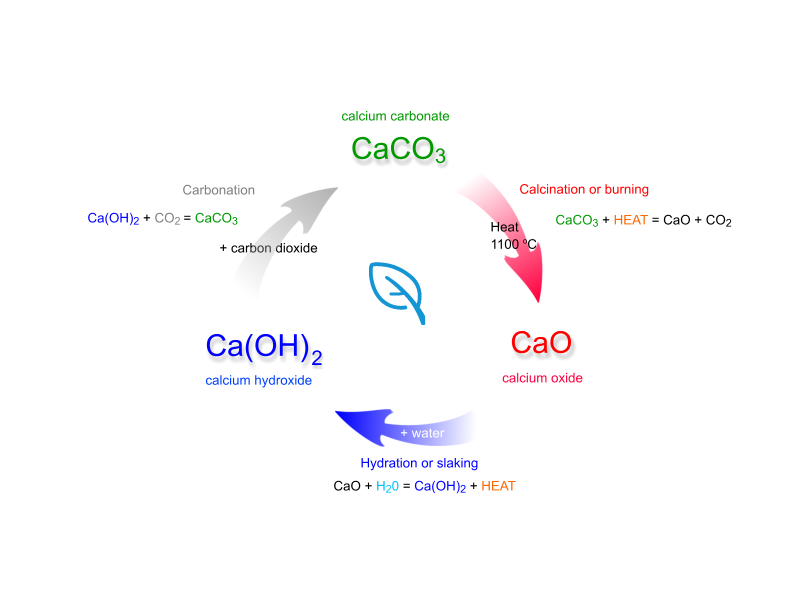
Limestone Cycle
Thermal decomposition
- calcium carbonate (s) > calcium oxide (s) + carbon dioxide (g)
- CaCO3 CaO CO2
Hydration
- calcium oxide (s) + water (l) > calcium hydroxide (s)
- CaO H2O Ca(OH)2
Dissolving
- calcium hydroxide (s) > calcium hydroxide (aq)
- Ca(OH)2 Ca(OH)2
Precipitation
- calcium hydroxide (aq) + carbon dioxide (g) > calcium carbonate (s) + water (l)
- Ca(OH)2 CO2 CaCO3 H2O
Limestone Cycle
Using Limestone
Quarrying Limestone (environmental problems)
- Huge ugly holes > perminently change landscape
- The processes make lots of noise and dust
- Destroys the habitats of animals
- Transporting limestone > lorries cause noise pollution
- Waste materials > unsightly tips
Using Limestone
- Cement factories create dust > breathing problems
- Energy is need to produce cement and quicklime (burning fossil fuels > pollution)
Benefits
- Limestone provides houses & roads. Limestone chemicals in dyes, paints, medicines
- Limestone products used to neutralise acidic soil, lakes & rivers (acid rain)
- Used to neutralise sulphur dioxide (causes acid rain) in power station chimneys
- Provides jobs and brings money to local economy > lead 2 local improvements
- Once complete > landscaping & restoration should take place
Limestone Products
Pros & Cons of Limestone Products
- Limestone is widely available and cheaper than granite & marble
- A fairly easy rock to cut
- Mostly more hard-wearing than marble, and looks attractive
- Concrete can be poured into moulds to make blocks/panels > quick and cheap way of constructing buildings > however, its extremely unattractive
- Limestone and contrete don't rot when wet.
- They can't be gnawed away by insects or rodents.
- They're fire-resistant
- Concrete doesn't corrode (unlike most metals)
- Has a fairly low tensile strength and can crack > it's stronger if it's reinforced with steel bars
Metals from Rocks
Unreactive metals (eg. gold) are found in their native state in the earth.
Ores are naturally occurring rocks that contain metal compounds with enough metal to make extraction worthwhile
Often the ore is an oxide of the metal (eg. main aluminium ore is bauxite > aluminium oxide)
The economics of metal extraction changes over time:
- Market price drops > may not be worth extracting. Market price increases > may be worth extracting more of it
- Technology improves > able to extract more metal from rock > may be more worthwhile extracting a metal previously not worth extracting
Metals are extracted from their ores chemically by REDUCTION or ELECTROLYSIS
- Some ores have to be concentrated before extraction (get rid of unwanted rock)
- Electrolysis can also be used to purify the extracted metal
- Occasionally metals can be extracted by using displacement reactions
Metals from Rocks (2)
Some Metals can be Extracted by REDUCTION with CARBON
When an ore is reduced, oxygen is removed from it
(eg. iron(III) oxide + carbon -> iron + carbon dioxide)
The position of the metal in the reactivity series determins whether it can be extracted by reduction with carbon
- Metals higher than carbon in the reactivity series have to extracted using electrolysis, which is expensive
- Metals below carbon in the reactivity series cane be extracted by reduction using carbon (eg. iron oxide is reduced in a blast furnace to get iron)
- This is because carbon can only take the oxygen away from metals which are less reactive than carbon itself
Reactivity Series
- Potassium, K More reactive
- Sodium, Na
- Calcium, Ca
- Magnesium, Mg
- Aluminium, Al
- CARBON, C
- Zinc, Zn
- Iron, Fe
- Tin, Sn
- Copper, Cu Less reactive
ABOVE CARBON -> Electrolysis
BELOW CARBON -> Reduction using carbon
Metals from Rocks (3)
Some Metals have to be Extracted by ELECTROLYSIS
- Metals more reactive than carbon > extracted using electrolysis of molten compounds (eg. Aluminium)
- Much more expensive than reduction (uses a lot of energy)
Copper is Purified by Electrolysis (impure copper is obtained through reduction with carbon > smelting)
- Impure copper doesn't conduct electricity well (not useful > mostly used in wiring)
- Electrolysis used to purify it (expensive) > pure copper > better conductor
Electrolysis (breaking down of substance with electricity)
- Requires a liquid to conduct the electricity (electrolyte > often metal salt solutions from ore / molten metal oxides)
- The electrolyte has free ions - conduct electricity
- Electrons are taken away by the (positive) anode and given away by the (negative) cathode. As ions gain/lose electrons they become atoms/molecules and are released
Metals from Rocks (4)
Example - Electrolysis used to get copper:
- Electrons are pulled off copper atoms at the anode, causing them to go into solution as Cu2+ ions
- These ions near the cathode gain electrons and turn back into copper atoms
- The impurities are dropped at the anode as a sludge, whilst pure copper atoms bond to the cathode
Metals from Rocks (5)
Extracting Copper from Solution using a Displacement Reaction
- More reactive metals react more vigorously than less reactive metals.
- If you put a reactive metal in a solution of a dissolved metal compound, the reactive metal will replace the less reactive metal in the compound.
- This is because the more reactive metal bonds more strongly to the non-metal bit of the compound and pushes out the less reactive metal.
- Eg. scrap iron can be used to displace copper from solution (useful > iron cheap, copper expensive). If some iron is put in a solution of copper sulphate, the more reactive iron will 'kick out' the less reactive copper from the solution. End up with iron sulphate solution and copper metal.
- copper sulphate + iron -> iron sulphate + copper
Copper-rich Ores are in Short Supply (limited supply, increasing demand > important to recycle)
- Scientists looking into new ways of extracting copper from low-grade ores/ waste from copper extraction
Metals from Rocks (6)
New Methods (less impact on environment, but slow)
- Bioleaching
- Using bacteria to separate copper from copper sulphide
- The bacteria get energy from the bond between copper and sulphur, separating copper from the ore in the process
- The leachate (solution produced by process) contains copper > extracted by filtering
- Phytomining
- Growing plants in soil that contains copper
- The plants can't use or get rid of the copper > it gradually builds up in the leaves
- The plants are harvested, dried and burned in a furnace
- The copper is collected from the ash left in the furnace
Metal Extraction Impacts
Social, economic & environmental Issues
- Pros - useful products made, provides jobs for locals, brings money to area > improve health and transport
- Cons - Noisey, ruins landscape, destroy habitats, mine shafts are dangerous
Recycling Metals is Important
- Mining & extracting metals uses a lot of energy (burning fossil fuels)
- Fossil fuels are running out, & they contribute to acid rain, global dimming and climate change
- Recycling metals takes a lot less energy than extracing & mining
- Energy is expensive > recycling saves money
- There is only a certain amount of metal in the earth (recycle to conserve)
- Recycling cuts down landfill > landfill takes space and pollutes the surroundings
Properties of Metals
- All metals have similar properties:
- Strong (hard to break)
- Can be bent/hammered into different shapes (malleable)
- Conduct heat and electricity well
Lots of everyday uses > strength & malleability (bridges & car bodies), great for conducting heat (saucepan base), conductivity (electrical wires)
Copper: good conductor of electricity, ductile, hard, strong, malleable, doesn't react with water. (used in plumbing)
Aluminium: corrosion-resistant, low density, pure aluminium > not strong, forms hard strong alloys. (used in aeroplanes)
Titanium: corrosion-resistant, low density, very strong. (used in replacement hips)
Metals > structurally useful, but some corrode when exposed to air and water > need to be protected (eg. paint). Corrosion > lose strength & hardness. When repeatedly strained > they get 'metal fatigue', leads to breaking (dangerous)
Alloys
Pure Iron Tends to be Too Bendy
- Iron from a blast furnace is only 96% iron, has impurities like carbon
- This impure iron is used as cast iron (in ornamental railings), too brittle for most uses
- All impurites are removed > pure iron, has a regular arrangement of identical atoms. The layers of atoms can slide over each other > making it soft and easily shaped (too bendy for most uses)
Most Iron is Converted into Steel (alloy)
- Low carbon steel > easily shaped (car bodies)
- High carbon steel > very hard, inflexible (blades for tools, bridges)
- Stainless steel (+ chronium) > corrosion-resistant (cutlery, containers)
Different elements have different sized atoms. If an element such as carbon is added to pure iron, the smaller carbon atoms will upset the layers of pure iron atoms > more difficult for them to slide > so alloys are harder.
Alloys (2)
Examples of Everyday Metal Alloys:
Bronze = Copper + Tin > bronze is harder than copper (statues & medals)
Cupronickel = Copper + Nickel > hard and corrosion-resistant (silver coins)
Gold alloys > pure gold is too soft > add metals like zinc, copper or silver to harden (jewellery)
Aluminium alloys > Al has a low density > alloyed to make it stronger (aircraft)
Fractional Distillation- Crude Oil
Crude oil is a fossil fuel > a mixture of hydrocarbons (fuels)
- The dif. hydrocarbon molecules in crude oil aren't chemically bonded together
- So they all keep their original properties (eg. condensing points)
- The parts of a mixture can be seperated by physical methods, eg. CRUDE OIL can be split into its seperate fractions by FRACTIONAL DISTILLATION. Each fraction contains molecules with a similar number of carbon atoms
Crude Oil is Split into Seperate Groups of Hydrocarbons
The fractioning column works continously, with heated crude oil piped in at the bottom. The vapourised oil rises up the column and the various fractions are constantly tapped off at the dif. levels where they condense.
Fractions: refinery gas > petrol > naphtha > kerosene > diesel > oil > bitumen.
Low > high, condensing points. Short > long molecule lengths.
Crude Oil
All the fractions of crude oil are hydrocarbons called alkanes (chains of carbon atoms surrounded by hydrogen atoms)
- Different alkanes have chains of dif. lengths.
- First four -> methane CH4, ethane C2H6, propane C3H8, butane C4H10
- Carbon atoms form four bonds, hydrogen atoms form one bond. If each atom forms bonds with as many other atoms as it can > then they're saturated
- Alkanes all have the general formula: CnH2n+2
Shorter molecules > low boiling point, low viscosity, high volatility, high flammability
- Volatility helps decide what the fraction is used for. Refinery gas > shortest molecules, lowest boiling point, gas at room temp. Ideal as bottled gas (stored under high pressure)
- Petrol fraction has longer molecules > higher boiling point, stored as liquid in car but easily vapourised and ignited
- Viscosity also helps decide the use. Really gloopy, viscous hydrocarbons > used for lubricating engine parts and covering roads
Crude Oil - Fuel
Crude oil fractions burn cleanly > good fuels. Most modern transport is fuelled by one. Parts of crude oil are also burned in central heating systems and in power stations (electricity).
Massive industry > as well as fuel, raw materials for chemicals & plastics. Alternatives: nuclear power and wind power to generate electricity, ethanol-powered cars, solar energy to heat water.
Oil fractions > readily available, generally cheap and easiest option. More reliable/safe than alternatives (wind, solar, nuclear)
Crude oil is non-renewable > it will run out. However, new oil reserves are found from time to time. New technology > easier and cheaper to extract more oil. We need to conserve, and come up with new alternatives/use renewable sources and adapt to using them.
- Environment: Oil spills can happen during transport in tankers > birds, sea otters, whales etc. get covered and poisoned by the oil. Disaster for local environment.
- You have to burn oil to get energy > major cause of global warming, acid rain and global dimming.
Environmental Problems
Power stations burn huge amounts of fossil fuels for electricity (cars also major culprit). Most fuels (like crude oil & coal) contain carbon and hydrogen. During combustion, carbon and hydrogen are oxidised > carbon dioxide and water vapour are released into atmosphere, energy also produced.
- Fuel containing sulphur impurities > sulphur dioxide released
- High temp. > oxides of nitrogen form
- Plenty of oxygen > all of fuel burns > complete combustion
- Not enough oxygen > some of fuel doesn't burn > partial combustion. Particulates of soot, unburnt fuel and carbon monoxide (poisonous) released.
Sulphur dioxide causes acid rain (mixes with clouds to form dilute suphuric acid, falls as acid rain). Same problem with oxides of nitrogen. Acid rain > acidic lakes > plants & animals die. Kills trees, damages limestone buildings & stone statues. Issues with human health problems. Benefits of electricity must be balanced against environmental impacts.
Can remove sulphur before burning (expensive, more energy & CO2). Replace petrol & diesel > low-sulphur versions. Intro Acid Gas Scrubbers to power stations. Reduce fossil fuel usage.
Environmental Problems (2)
Level of CO2 in atmosphere is increasing (burning fossil fuels) > global warming. Changes rainfall patterns, could cause severe flooding > polar ice caps melting
In some areas nearly 25% less sunlight has been reaching the surface of the earth compared to 50 years ago > global dimming. Burning fossil fuels > particles of soot and ash produced > reflect sunlight back into space. Not all scientists believe this.
- Alternative fuels
- Ethanol: produced by fermenting plants (biofuel) > power cars.
- Pros: CO2 released was taken in by plant as it grew, 'carbon neutral'
- Cons: engines have to convert to use, not widely available, may replace food farming
- Biodiesel: a biofuel from vegetable oils > mix with diesel for vehicles
- Pros: 'carbon neutral', engines don't need converting, produces less SO4 & particulates
- Cons: can't make enough to replace diesel, expensive, may replace food farming
- Hydrogen gas: from electrolysis of water > power vehicles
- Pros: H2 combines with O2 to produce just H2O > very clean fuel
- Cons: need special expensive engine, H2 not widely available, hard to store, need energy
Related discussions on The Student Room
- Maths for economists - constrained maximisation questoin »
- Physics Question help »
- circle question help »
- Learning a 2nd language »
- Energy dissipated »
- Isaac Senior Physics Challenge 2024 »
- best way to revise maths »
- How do I derive the order of growth in this code? »
- Senior Physics Challenge 2022 »
- Edexcel GCSE Chemistry (Paper 1) »
Comments
Report
Report